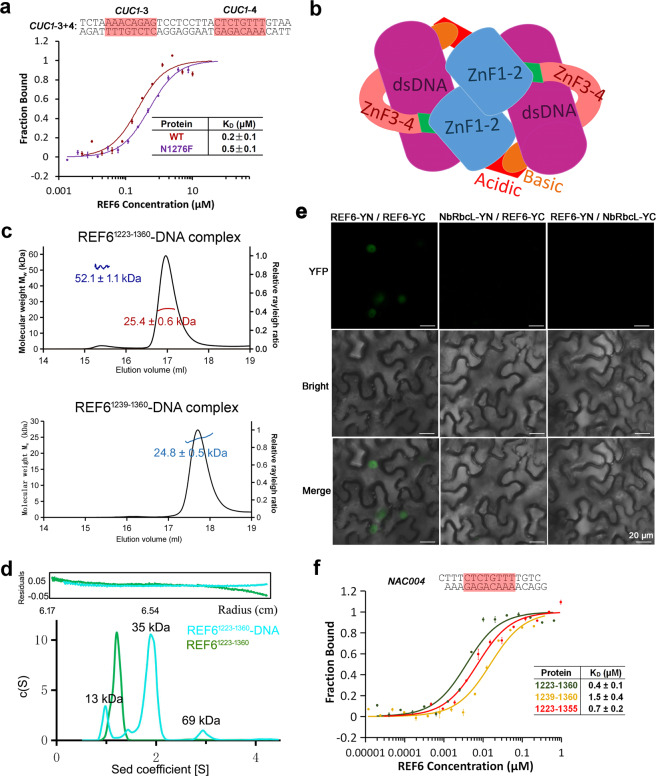
Fig. 7. Cooperativity of REF6 enhances its binding to dsDNA.
a The binding affinity of the mutation N1276F at the interface with CUC1-3 + 4 is lower than the WT. b Model of a heterotetrameric REF6-DNA complex. c The SEC-MALS results show the oligomeric states of REF61223–1360-DNA complex and REF61239–1360-DNA complex at 0.8 mg mL−1. d The oligomeric state of REF61223–1360 by analytical ultracentrifugation assays at 1.0 mg mL−1. The c(s) distribution from SV analysis is shown. e BiFC assays showing self-association of the full-length REF6 in vivo. An unrelated chloroplast protein encoded by NbRbcL was used as a negative control62. f Loss of the acidic region or basic region obviously decreased the DNA-binding affinity of REF6 with NAC004 dsDNA fragments.