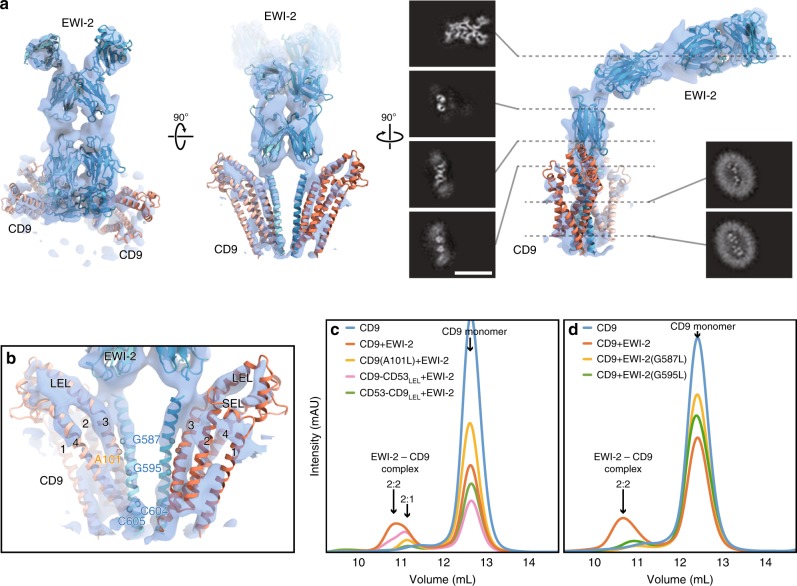
Fig. 4. Cryo-EM structure of CD9 in complex with EWI-2.
a Cryo-EM density map of the Fab-CD9-EWI-2 complex. The density for the Fab fragment was somewhat disordered (Supplementary Fig. 6). Homology models of the Ig-like domains of EWI-2 were constructed with the PHYRE2 server80 and roughly fitted into the density. The TM helices and the two extracellular loops (SEL and LEL) of the CD9 crystal structure are separately fitted into the density. The density for the detergent micelle is omitted for clarity. Black panels show sections of the density, demonstrating the C2 symmetry in the TM region and the proximal Ig-like domain. Scale bar, 100 Å. b Close-up view of the TM region. CD9 adopts a semi-open conformation with a slight rearrangement of the LEL, as compared to the crystal structure (Supplementary Fig. 6). The residues in the EWI-2 membrane-spanning region were modeled according to the mutant analyses in Fig. 2c, d. c, d Complex formation assays for CD9 mutants (c) and EWI-2 mutants (d). GFP-labeled CD9 and EWI-2 were co-expressed in HEK cells, and complex formation was analyzed by FSEC. The slight peak shift toward the smaller molecular weight indicates the complex formation with 2:1 stoichiometry.