Figure 1.
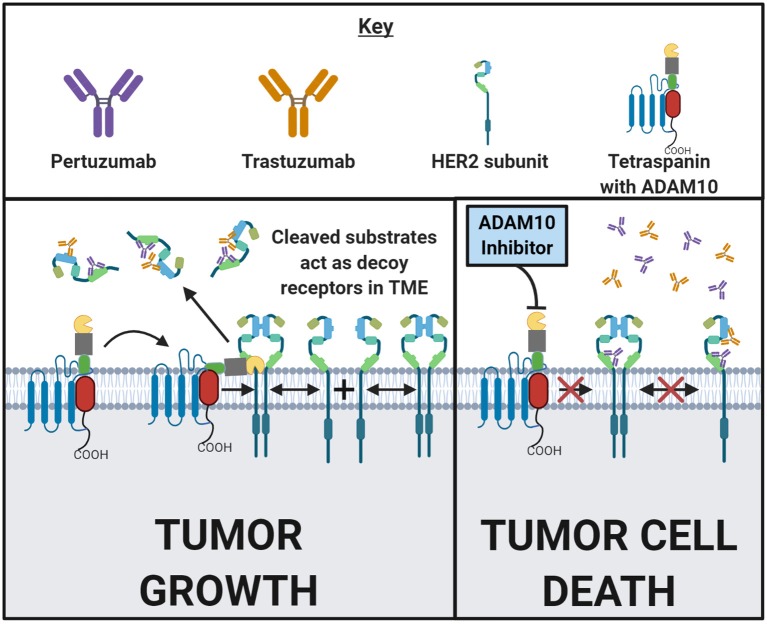
ADAM10 mediates cell surface cleavage of a large repertoire of substrates that can promote a pro-growth environment in malignant tumors. Some of these ADAM10 substrates, such as HER2, are targeted by FDA approved drugs. Following HER2 subunit dimerization with HER- family subunits, and upon ligand-binding or autoactivation, a pro-growth signal cascade is initiated which then drives malignancy. Trastuzumab and pertuzumab are both monoclonal antibodies that bind to two distinct epitopes of HER2 to prevent homodimerizaton and heterodimerization, respectively. In addition to inhibiting dimerization, tumor cells with bound antibody have an increased likelihood of succumbing to antibody dependent cellular cytotoxicity (ADCC) or opsonization. In the left and right panels above, a HER2-positive cancer cell surface in the presence of trastuzumab and pertuzumab is depicted. In the left panel, ADAM10 cleaves the extracellular domain of HER2 from the cell surface into the tumor microenvironment (TME). These cleaved domains then act as decoy receptors, decreasing the amount of trastuzumab or pertuzumab that binds to the tumor cell. The likelihood of ADCC or opsonization is now decreased. In the right panel, treatment with trastuzumab or pertuzumab is combined with the inhibition of ADAM10. Inhibiting ADAM10 results in less HER2 cleavage which reduces the amount of decoy receptors in the TME. Decreasing the decoy receptors in the TME and increasing the amount of HER2 on the tumor cell might enhance trastuzumab's and/or pertuzumab's antitumor effects. This general concept can be applied to other ADAM10 substrates in other disease states. Made in ©BioRender - biorender.com.