FIGURE 4.
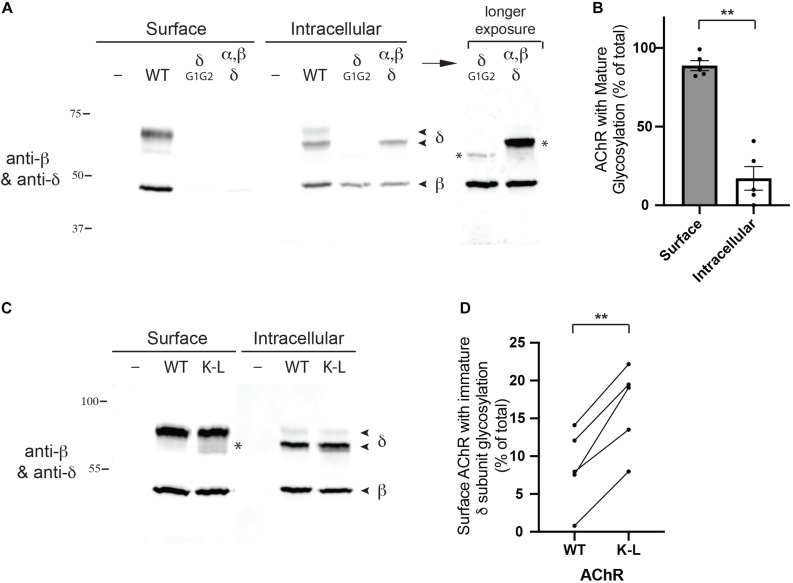
Mutation of the Golgi retention motif increases surface AChR with immature glycosylation. (A) HEK cells were transfected with wild type AChR subunits (α, β, ε, and an epitope-tagged δ subunit), or with AChR containing δ subunit with mutated N-linked glycosylation sites. We then sequentially isolated surface and intracellular AChR and immunoblotted the isolates for the β and δ subunits. We found that surface AChR contained a higher molecular weight form of δ subunit compared to intracellular AChR (∼68 cf. 62 kD), which has been shown to be due to modification of N-linked oligosaccharides during transit through the Golgi complex. Consistent with this, AChR containing δ with mutated glycosylation sites (δG1G2), or only α, β and δ subunits were largely retained in the intracellular receptor pool and contained only lower molecular weight forms of δ (marked by asterisks in longer exposure image). (B) Quantification shows that surface AChR contains largely high molecular weight δ subunit with mature glycosylation (89% of total). In contrast, intracellular AChR contains only 17% high molecular weight δ subunit, with the vast majority (83%) being low molecular weight δ subunit with immature glycosylation (**p < 0.005; paired t test; n = 5 experiments; error bars = SEM). (C) HEK cells were transfected with wild type AChR or AChR containing combined βK353L and δK351L mutations (K–L), and surface and intracellular AChR were sequentially isolated and immunoblotted with anti-β and δ subunit antibodies. Compared to wild type AChR, mutant AChR contained significantly more low molecular weight delta subunit with immature glycosylation (asterisk). (D) Quantification showing the percentage of WT and K–L surface AChR with immature δ subunit glycosylation, with each pair of data points representing a separate transfection experiment. Combined βK353L/δK351L mutations increased the amount of surface AChR with immature glycosylation by twofold, from 8 to 16% (**p < 0.005; paired t-test; n = 5 experiments).