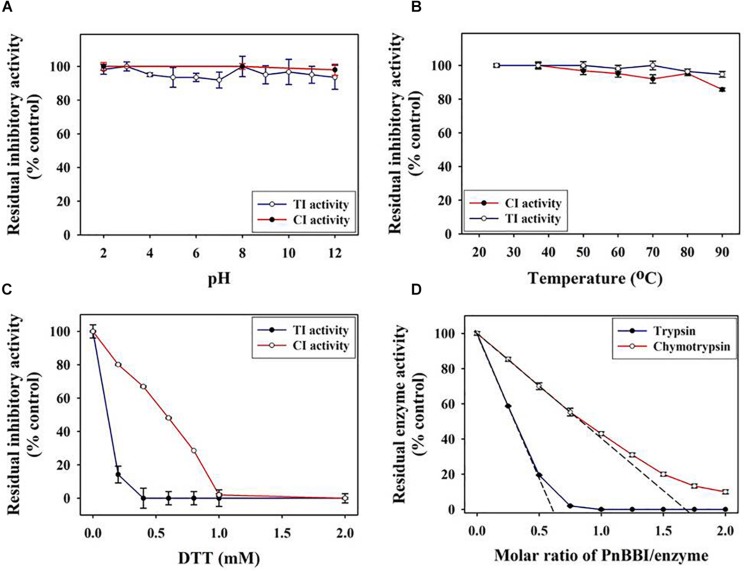
FIGURE 5.
Functional stability of PnBBI. The TI and CI activities of PnBBI were monitored at a broad range of (A) pH; (B) temperature and (C) reducing agent Dithiothreitol (DTT). The amount of PnBBI equivalent to one TI or CI units was incubated at different pH, temperatures or DTT concentration and residual inhibitory activities against trypsin and chymotrypsin was monitored using respective substrates as described in methods (Section “Inhibitory Activity of PnCPI and PnBBI”); (D) Titration curve of trypsin and chymotrypsin inhibition by PnBBI. A fixed amount of enzyme (1 μM trypsin and/or 2 μM chymotrypsin) was mixed with different concentrations of PnBBI and the residual protease activity was determined. The x-intercept where the corresponding proteases are completely inhibited by the inhibitor was the molar binding ratio. The data represented is mean ± SE of three biological replicates.