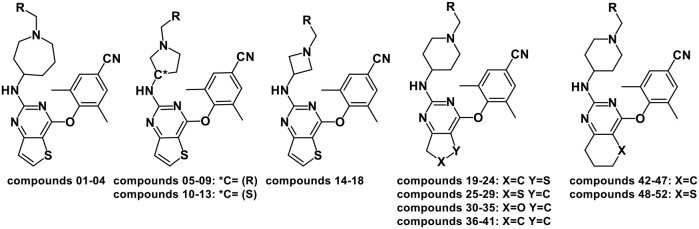
Table 1.
Chemical structures of DHPYs and their actual and predicted activities as HIV-1 NNRTIs.
 | |||||||
|---|---|---|---|---|---|---|---|
| CoMFA | CoMSIA | ||||||
| No. | R | EC50 (nM) | Actual pEC50 | Predicted pEC50 | Residual | Predicted pEC50 | Residual |
| 01b | 4-SO2NH2-Ph | 2.20 | 8.658 | 8.728 | 0.070 | 8.672 | 0.014 |
| 02 | 3-CONH2-Ph | 10.3 | 7.987 | 8.043 | 0.056 | 8.014 | 0.027 |
| 03 | 4-SO2CH3-Ph | 9.98 | 8.001 | 7.919 | −0.082 | 7.992 | −0.009 |
| 04a | 4-pyridinyl | 31.7 | 7.499 | 7.528 | 0.029 | 7.392 | −0.107 |
| 05b | 4-SO2NH2-Ph | 8.69 | 8.061 | 8.046 | −0.015 | 8.076 | 0.015 |
| 06 | 4-CONH2-Ph | 10.4 | 7.983 | 8.042 | 0.059 | 8.025 | 0.042 |
| 07 | 3-CONH2-Ph | 41.3 | 7.384 | 7.360 | −0.024 | 7.365 | −0.019 |
| 08 | 4-SO2CH3-Ph | 13.9 | 7.857 | 7.923 | 0.066 | 7.860 | 0.003 |
| 09a | 4-pyridinyl | 16.0 | 7.796 | 7.500 | −0.296 | 7.830 | 0.034 |
| 10 | 4-SO2NH2-Ph | 104 | 6.983 | 7.083 | 0.100 | 7.004 | 0.021 |
| 11 | 4-CONH2-Ph | 55.7 | 7.254 | 7.164 | −0.090 | 7.242 | −0.012 |
| 12 | 4-SO2CH3-Ph | 50.7 | 7.295 | 7.214 | −0.081 | 7.275 | −0.020 |
| 13b | 4-pyridinyl | 16.7 | 7.777 | 7.843 | 0.066 | 7.781 | 0.004 |
| 14 | 4-SO2NH2-Ph | 4.53 | 8.344 | 8.251 | −0.093 | 8.335 | −0.009 |
| 15 | 4-CONH2-Ph | 4.76 | 8.322 | 8.317 | −0.005 | 8.304 | −0.018 |
| 16 | 3-CONH2-Ph | 8.95 | 8.048 | 7.954 | −0.094 | 8.053 | 0.005 |
| 17a | 4-SO2CH3-Ph | 207 | 6.684 | 6.860 | 0.176 | 7.329 | 0.645 |
| 18b | 4-pyridinyl-Ph | 2.21 | 8.656 | 8.690 | 0.034 | 8.680 | 0.024 |
| 19 | 4-SO2NH2-Ph | 4.3 | 8.367 | 8.468 | 0.101 | 8.467 | 0.100 |
| 20a | 4-CONH2-Ph | 4.8 | 8.319 | 8.490 | 0.171 | 8.328 | 0.009 |
| 21 | 4-SO2CH3-Ph | 5.9 | 8.229 | 8.197 | −0.032 | 8.238 | 0.009 |
| 22b | 4-pyridinyl | 2.6 | 8.585 | 8.613 | 0.028 | 8.637 | 0.052 |
| 23a | 4-NO2-Ph | 8.0 | 8.097 | 8.103 | 0.006 | 8.226 | 0.129 |
| 24 | 3-CONH2-Ph | 27.7 | 7.558 | 7.500 | −0.058 | 7.547 | −0.011 |
| 25a | 4-SO2NH2-Ph | 37.2 | 7.429 | 7.494 | 0.065 | 7.263 | −0.166 |
| 26a, b | 4-SO2Me-Ph | 3.8 | 8.420 | 8.406 | −0.014 | 7.714 | −0.706 |
| 27 | 4-NO2-Ph | 11.5 | 7.939 | 7.902 | −0.037 | 7.898 | −0.041 |
| 28 | 4-NH2-Ph | 8.4 | 8.076 | 7.889 | −0.187 | 8.067 | −0.009 |
| 29 | 4-NHSO2Me-Ph | 11.2 | 7.951 | 8.043 | 0.092 | 7.987 | 0.036 |
| 30 | 4-SO2NH2-Ph | 2.8 | 8.553 | 8.562 | 0.009 | 8.543 | −0.010 |
| 31b | 4-CONH2-Ph | 1.6 | 8.796 | 8.768 | −0.028 | 8.812 | 0.016 |
| 32 | 4-SO2CH3-Ph | 1.9 | 8.721 | 8.677 | −0.044 | 8.629 | −0.092 |
| 33 | 4-pyridinyl | 2.3 | 8.638 | 8.701 | 0.063 | 8.736 | 0.098 |
| 34 | 4-NO2-Ph | 7.4 | 8.131 | 8.075 | −0.056 | 8.178 | 0.047 |
| 35a | 3-CONH2-Ph | 7.8 | 8.108 | 7.971 | −0.137 | 8.238 | 0.130 |
| 36b | 4-SO2NH2-Ph | 1.1 | 8.959 | 8.867 | −0.092 | 8.941 | −0.018 |
| 37 | 4-CONH2-Ph | 6.1 | 8.215 | 8.387 | 0.172 | 8.270 | 0.055 |
| 38a | 4-SO2CH3-Ph | 4.9 | 8.310 | 8.603 | 0.293 | 8.479 | 0.169 |
| 39 | 4-pyridinyl | 1.8 | 8.745 | 8.642 | −0.103 | 8.452 | −0.293 |
| 40 | 4-NO2-Ph | 14.5 | 7.839 | 7.953 | 0.114 | 7.858 | 0.019 |
| 41a | 3-CONH2-Ph | 2.0 | 8.699 | 8.060 | −0.639 | 8.437 | −0.262 |
| 42 | 4-SO2NH2-Ph | 6.0 | 8.222 | 8.145 | −0.077 | 8.169 | −0.053 |
| 43b | 4-CONH2-Ph | 6.0 | 8.222 | 8.182 | −0.040 | 8.218 | −0.004 |
| 44a | 4-SO2CH3-Ph | 8.0 | 8.097 | 8.278 | 0.181 | 8.308 | 0.211 |
| 45 | 4-pyridinyl | 8.6 | 8.066 | 8.033 | −0.033 | 8.188 | 0.122 |
| 46 | 4-NO2-Ph | 77.4 | 7.111 | 7.330 | 0.219 | 7.106 | −0.005 |
| 47a | 3-CONH2-Ph | 6.5 | 8.187 | 7.792 | −0.395 | 8.369 | 0.182 |
| 48b | 4-SO2NH2-Ph | 2.7 | 8.569 | 8.588 | 0.019 | 8.540 | −0.029 |
| 49 | 4-CONH2-Ph | 3.0 | 8.523 | 8.523 | 0.000 | 8.434 | −0.089 |
| 50 | 4-SO2CH3-Ph | 3.9 | 8.409 | 8.453 | 0.044 | 8.451 | 0.042 |
| 51 | 4-NO2-Ph | 8.6 | 8.066 | 8.024 | −0.042 | 8.056 | −0.010 |
| 52a | 3-CONH2-Ph | 5.1 | 8.292 | 8.087 | −0.205 | 8.429 | 0.137 |
Test set compounds used for 3D-QSAR models.
The compounds used for pharmacophore models.
CoMFA, comparative molecular field analysis; CoMSIA, comparative molecular similarity indices analysis; DHPY, dihydrofuro[3,4-d]pyrimidine; NNRTIs, non-nucleoside reverse transcriptase inhibitors.
