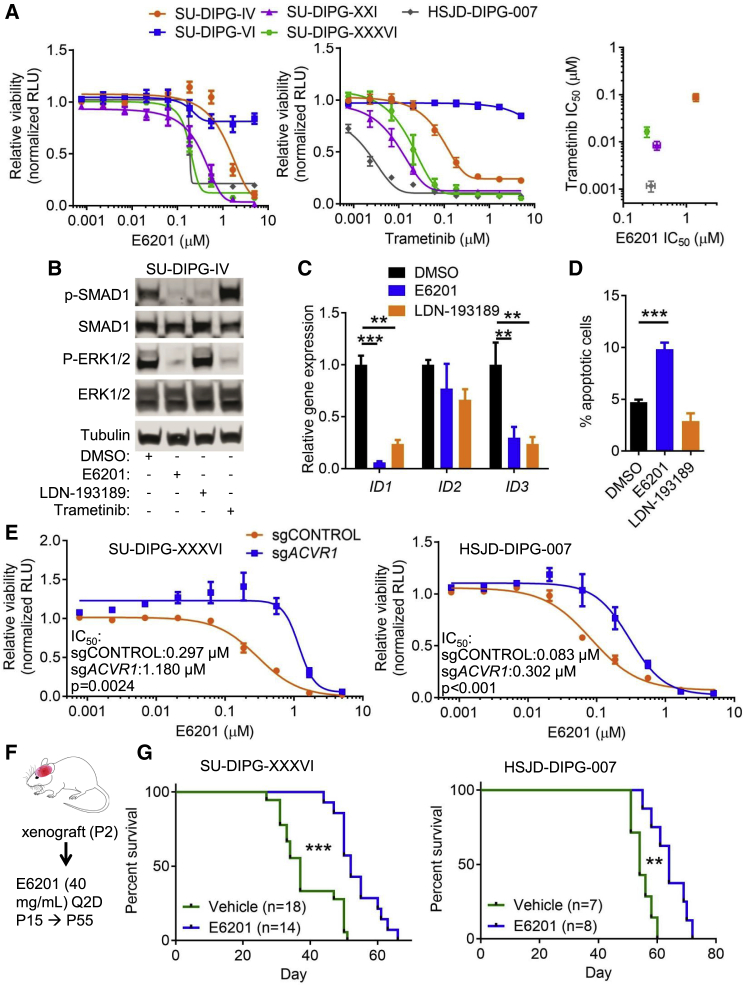
Figure 7.
E6201 Impairs ACVR1 Mutant DIPG Cell Viability and Tumorigenicity
(A) Relative ATP-dependent luminescence activity in the indicated cell lines, exposed to increasing concentrations of E6201 (left), or Trametinib (middle). n = 3 experiments. Right: E6201 and Trametinib IC50 in ACVR1 mutant cell lines.
(B) Western blot of lysates from SU-DIPG-IV cells treated for 24 h with DMSO, 2 μM E6201, 1 μM LDN-193189, or 0.1 μM Trametinib, probed the indicated antibodies.
(C) Expression of ID1, ID2, and ID3, measured by qPCR, in SU-DIPG-IV cells treated for 24 h with DMSO, 2 μM E6201, or 1 μM LDN-193189. n = 3 experiments.
(D) Percentage of AnnexinV-positive cells in SU-DIPG-IV cells treated for 48 h with DMSO, 2 μM E6201, or 1 μM LDN-193189. n = 3 experiments.
(E) Relative ATP-dependent luminescence activity in SU-DIPG-XXXVI (left) or HSJD-DIPG-007 (right) cells transduced with lentiviruses encoding the indicated sgRNAs, and exposed to increasing concentrations of E6201. Data were normalized to the vehicle-treated condition for each sgRNA. n = 3 experiments.
(F) Experimental design and treatment protocol for assessment of E6201 in DIPG xenograft mouse models.
(G) Survival curves of NSG mice xenografted at postnatal day 2 with 2 × 105 SU-DIPG-XXXVI (left), or HSJD-DIPG-007 (right) cells, treated with E6201 or vehicle control (30% Captisol).
In all panels, mean + SEM is shown. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; assessed by repeated-measures ANOVA with Tukey multiple comparisons tests (C and D), nonlinear regression analysis (E) or Mantel-Cox tests (G). See also Figure S7.