Abstract
To warrant potential clinical testing, the equine anti-SARS–CoV F(ab′)2 requires evaluation in as many animal models as possible and a safety test in a primate model. In this study, we evaluated the pharmacokinetics, tolerance and immunity of this kind of antibody in macaques and rats. Results showed that the F(ab′)2 fragments had a normal metabolism in injected animals. The general physiological indexes did not differ between animals injected with anti-SARS–CoV F(ab′)2 or saline. However, a mild inflammatory response in local injection site and a moderate immune response against this antibody in the successively injected animals were observed, which however recovered 3 weeks after the last injection. The antibody titring from 1:100 to 400 against the equine anti-SARS–CoV F(ab′)2 in the inoculated hosts could be detected at week 2 during the successive injections of the equine F(ab′)2. The considerable safety of this antibody used in primates and the fact that the immune system of the host can be motivated by post-injection of the F(ab′)2 indicate that this type of anti-SARS–CoV antibody can be used for prevention and treatment of SASR, especially at the early stage of this virus infection. In addition, it can also provide the precious time for the combined use of other anti-SARS–CoV agents such as antiviral drug and vaccine.
Abbreviations: SARS: Severe acute respiratory syndrome, TCID50: 50%, tissue culture infective doses, t1/2: The terminal half-lives, AUC: The areas under the plasma concentration time curves, CL/F: The total clearance rates, MRT: Mean residence time, Vss: Apparent volume of distribution for steady state, Kel: Elimination rate constant.
Keywords: Equine, F(ab′)2, SARS–CoV, Pharmacology, Immunogenicity
1. Introduction
The prevention and treatment of severe acute respiratory syndrome (SARS) includes several strategies, including vaccines currently under development [1], [2], [3], [4], antiviral drugs and passive transfer of antibodies. Some antiviral agents such as interferons, ribavirin, and HIV protease inhibitors have already shown promising results [5], [6], [7], though they were usually used empirically during the 2002–2003 SARS outbreak.
Passive immunity has been applied in prevention and treatment of infectious diseases for a long time [8]. The practice of administering polyclonal immunoglobulins from hyperimmune sera of animal or human origin have a 100-year history of being effective against some viruses [9], [10], [11], [12], [13], providing another candidate strategy for protection against SARS–CoV infection. Yo and colleagues found that infusion of convalescent plasma demonstrated beneficial clinical outcomes in SARS patients [14]. Subbarao et al. verified that passive transfer of SARS–CoV specific antisera reduces pulmonary viral titres in mice infected with SARS–CoV [15], indicating that hyperimmune sera against SARS–CoV could protect against this viral infection.
Equine antiserum has been applied as an antiviral regimen to control rabies [16], HBV [11], [13], and HIV [9], [12] infections. We have generated equine anti-SARS–CoV F(ab′)2 fragments, which were shown to neutralize effectively SARS–CoV in vitro and in vivo in a BALB/C mouse model [17], aged mouse model [18], Golden hamster [19] and Chinese hamster model [20]. However, before any possible clinical applications, this antibody has to be tested rigorously in as many animal models as possible to insure its efficacy and safety.
Herein, this study was designed to evaluate the safety and pharmacokinetics of this antibody in the rat and macaque in order to provide valuable experimental data for potential clinical use of this type of anti-SARS–CoV antibody.
2. Materials and methods
2.1. Virus, antibody and animals
SARS–CoV (strains BJ-01 Genbank accession number AY278488) was maintained in the Institute of Microbiology Epidemiology, AMMS, China. The viral titre was 1.13 × 107 of 50% tissue culture infective doses (TCID50)/mL. All operations with SARS–CoV were performed in the Bio-Safety Level 3 (BSL-3) laboratory. The equine anti-SARS–CoV F(ab′)2 against the above strain of SARS–CoV was endotoxin free and prepared as described in our previous publication [17].
The macaques and rats used in this study were provided by the Animal Centre of Academy of Military Medical Sciences, Beijing, China. 27 macaques weighing 4.8 ± 0.8 kg each were fed individually, among which 9 were used for pharmacokinetic study and 18 in safety tests. Approval for animal experiments was obtained from the institutional animal welfare committee.
2.2. Histopathology
Routine histology assay was done as described by Subbarao et al. [15].
2.3. Pharmacokinetic study of equine anti-SARS–CoV F(ab′)2
Macaques and rats were used for pharmacokinetic studies. Macaques were divided into 3 dose groups to receive: 1, 3 and 10 mg of F(ab′)2 per kilogram of body weight, respectively. The F(ab′)2 was labelled with 125I and the specific activity of 125I-labelled F(ab′)2 was 84.8 kBq/μg. The animals in each dose group were i.v. injected with 8.5 MBq of 125I-labelled F(ab′)2, but the specific activity between each group was different. In addition, a successive administration group was set up. Animals would be i.v. injected with 3 mg/kg F(ab′)2 successively at the indicated time point. Animals before injection and at 0.083, 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 96, 144 and 168 h after injection were bled via caudal vein. Then the total sera γ-radioactivity was measured. For the 3 mg/kg successive administration group, the second injection was conducted on the 7th day after the first injection and the animals were then injected i.v. every week. These animals would be bled at same time point after the fourth injection and the total sera radioactivity was measured as above.
Rats were i.v. injected with 3 mg/kg of 125I-labelled equine F(ab′)2 with 4.48 kBq/μg of specific radioactivity and 13.44 MBq/kg of radioactivity dose. Animals before injection and at 0.083, 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 96, 144, 168, 192 and 216 h after injection were bled via caudal vein. Then the total sera γ-radioactivity was measured as described above for macaques.
2.4. Safety and immunogenicity test
18 macaques were randomly divided into 3 groups. Each animal received an i.v. injection of saline, or 0.5 and 5 mg/2 mL/kg body weight of anti-SARS–CoV F(ab′)2, respectively. The animals were successively injected once a day for 4 weeks. Then 4 macaques in each group were killed and the pathology of individual organs and tissues was studied. The remaining animals were thereafter observed further for 3 weeks without injections, followed by sacrifice and pathological investigation. All animal behavior, body weight, body temperature, electrocardiogram, hematologic indexes, hematologic biochemical indicators, urine indexes and sera antibody were observed.
2.5. Statistical analysis
Statistical analyses were performed by 1-way ANOVA and/or multiple comparison (Scheffe) and Student's t-test. All graphs represent the mean ± SEM.
3. Results
3.1. Pharmacokinetics of equine anti-SARS–CoV F(ab′)2 in macaques
After injection of anti-SARS–CoV F(ab′)2 into a macaque, the serum F(ab′)2 concentration decreased gradually. However, at each time point, the higher dose of F(ab′)2 was injected, the higher sera F(ab′)2 concentration would be retained (Fig. 1A). After i.v. injection of 1, 3 and 10 mg/kg of 125I-labelled F(ab')2, the terminal half-lives (t1/2) for total sera radioactivity were all between 41–46 h (Table 1 ). In accordance with the 1:3:10 ratio of injected dose of the antibody, the ratio of growth rate of the areas under the plasma concentration time curves (AUC(0–168 h)) was 1:2.8:8.9. The total clearance rates (CL/F) were not significantly different between each dose group, indicating the linear pharmacokinetics of the three injected doses of antibody in this study (Table 1).
Fig. 1.
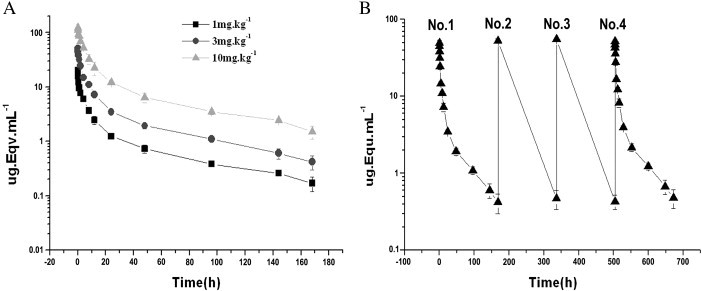
Concentration–time curve of the i.v. injected 125I-labelled F(ab′)2 in macaques. Panel A, the total serum F(ab′)2 concentration (radioactivity)–time course. The injected dose of the 125I-labelled antibody is indicated. Panel B, the total serum F(ab′)2 concentration–time course of successive injection. The order of injection is indicated. The experiments were repeated three times.
Table 1.
Pharmacokinetic parameters of total sera radioactivity after i.v. injection of various doses of 125I-labelled F(ab′)2 in macaques
| Parameters | Unit | Injected dose |
||
|---|---|---|---|---|
| 1 mg/kg | 3 mg/kg | 10 mg/kg | ||
| AUC(0–168 h) | μg Equ h mL− 1 | 159.0 ± 15.2a3,b3 | 446.52 ± 36.0c3 | 1419.9 ± 165.7 |
| AUC(0–∞) | μg Equ h mL− 1 | 170.5 ± 18.0a2,b2 | 471.7 ± 43.3c2 | 1522.4 ± 184.0 |
| AUC(168 h–∞) | μg Equ h mL− 1 | 11.4 ± 4.4a1,b1 | 25.2 ± 7.3c1 | 102.5 ± 31.8 |
| AUC(168 h–∞)% | % | 6.6 ± 2.1 | 5.3 ± 1.0 | 6.7 ± 1.6 |
| MRT (h) | H | 35.7 ± 1.8 | 33.7 ± 1.5 | 36.4 ± 1.7 |
| CL/F | mL kg− 1 h− 1 | 5.9 ± 0.7 | 6.4 ± 0.6 | 6.6 ± 0.9 |
| VSS | mL kg− 1 | 210.8 ± 18.1 | 215.3 ± 10.3 | 241.3 ± 28.2 |
| t1/2 (h) | H | 46.1 ± 3.4 | 41.2 ± 1.2 | 45.9 ± 3.0 |
| Kel | 1/h | 0.0151 ± 0.0011 | 0.0168 ± 0.0005 | 0.0151 ± 0.0010 |
a1, a2 or a3: 1 mg/kg vs 3 mg/kg group; intergroup t-test, p < 0.05, p < 0.01 or p < 0.01, respectively.
b1, b2 or b3: 1 mg/kg vs 10 mg/kg; paring t-test, p < 0.05, p < 0.01 or p < 0.01, respectively.
c1, c2 or c3: 3 mg/kg vs 10 mg/kg; paring t-test, p < 0.05, p < 0.01 or p < 0.01, respectively.
When the 3 mg/kg body weight of anti-SARS–CoV F(ab′)2 was i.v. injected successively, the total sera radioactivity did not show any difference between the 1st and the 4th injection (Fig. 1B, p > 0.05), suggesting that multiple i.v. injections at the indicated time points did not exert influence on the concentration–time curve of sera equine F(ab′)2. The t1/2 of total anti-SARS–CoV F(ab′)2 concentration in the sera was not different between the 1st and the 4th injection (p > 0.05) (Table 2 ). There was also no significant difference in other pharmacokinetic parameters of the 1st and the 4th injection of the antibody such as CL/F, mean residence time (MRT), apparent volume of distribution for steady state (Vss), AUCs and elimination rate constant (Kel) (Table 2).
Table 2.
Comparison of pharmacokinetic parameters of sera 125I-labelled F(ab′)2 between the 1st and the 4th i.v. injection in macaques
| Parameters | Unit | Total sera radioactivity |
p valuea | |
|---|---|---|---|---|
| The 1st injection | The 4th injection | |||
| AUC(0–24 h) | μg Equ h mL− 1 | 446.52 ± 36.0 | 504.1 ± 40.8 | 0.1421 |
| AUC(0–∞) | μg Equ h mL− 1 | 471.7 ± 43.3 | 533.8 ± 51.1 | 0.1853 |
| AUC(24–∞) | μg Equ h mL− 1 | 25.2 ± 7.3 | 29.7 ± 10.3 | 0.5697 |
| AUC(24 h–∞)% | % | 5.3 ± 1.0 | 5.5 ± 1.3 | 0.8420 |
| MRT (h) | H | 33.7 ± 1.5 | 33.9 ± 1.4 | 0.9312 |
| CL/F | mL kg− 1 h− 1 | 6.4 ± 0.6 | 5.9 ± 0.5 | 0.1707 |
| VSS | mL kg− 1 | 215.3 ± 10.3 | 190.9 ± 10.1 | 0.0532 |
| t1/2 (h) | H | 41.2 ± 1.2 | 42.6 ± 2.9 | 0.5137 |
| Kel | 1/h | 0.0168 ± 0.0005 | 0.0163 ± 0.0011 | 0.5317 |
1st vs 4th total sera radioactivity.
3.2. Pharmacokinetics of equine anti-SARS–CoV F(ab′)2 in rats
The antibody showed a similar sera concentration–time course in rats injected with 3 mg/kg body weight of anti-SARS–CoV F(ab′)2 as that observed in macaques (data not shown). The corresponding parameters are shown in Table 3 . The t1/2 of this antibody in rats was 56.3 ± 4.0 h for total radioactivity (Table 3), which was higher than that seen in macaques (Table 2).
Table 3.
Pharmacokinetics of i.v. injected 3 mg/kg 125I-labelled F(ab′)2 in rat sera
| Parameter | Unit | Total radioactivity |
|---|---|---|
| AUC(0–216 h) | ng h mL− 1 | 62169.6 ± 3712.7 |
| AUC(0–∞) | ng h mL− 1 | 62444.1 ± 3713.6 |
| AUC(216–∞) | ng h mL− 1 | 274.5 ± 25.5 |
| AUC(216–∞)% | % | 0.44 ± 0.05 |
| MRT | H | 38.1 ± 0.6 |
| CL/F | L h− 1 kg− 1 | 0.021 ± 0.001 |
| VSS | L kg− 1 | 0.79 ± 0.05 |
| t1/2 | H | 56.3 ± 4.0 |
| Kel | h− 1 | 0.0124 ± 0.0009 |
3.3. Tolerance and immune response to equine anti-SARS–CoV F(ab′)2 in macaques
All general physiological indexes did not differ between animals injected with anti-SARS–CoV F(ab′)2 or saline. The body weight, body temperature, electrocardiogram, hematology and biochemical indexes were all normal. However, 9/12 antibody-injected animals developed skin erythema with the radius of 3 cm at the injection site after 8 successive injections. Also, two and more weeks after the last injection, antibodies against the equine F(ab′)2 could be detected in the macaque sera. The highest anti-F(ab′)2 titre observed was 1:1600, 3 weeks after the last injection (Table 4 ).
Table 4.
Antibody raised in macaques injected with equine anti-SARS–CoV F(ab′)2
| Ab dose | Animal no. | Anti-F(ab')2 antibody titre |
||
|---|---|---|---|---|
| 2 weeks | 4 weeks | 7 weeks | ||
| Control | 151–156 | ND | ND | ND |
| 0.5 mg/kg | 157 | 1:100 | ND | 1:400 |
| 158 | 1:400 | 1:50 | ⁎ | |
| 159 | 1:400 | 1:400 | ⁎ | |
| 160 | 1:100 | 1:400 | 1:800 | |
| 161 | 1:400 | 1:400 | ⁎ | |
| 162 | 1:400 | 1:800 | ⁎ | |
| 5 mg/kg | 163 | 1:200 | 1:100 | ⁎ |
| 164 | ND | 1:100 | ⁎ | |
| 165 | 1:100 | 1:200 | 1:1600 | |
| 166 | 1:200 | 1:400 | ⁎ | |
| 167 | 1:100 | 1:50 | 1:1600 | |
| 168 | 1:200 | 1:800 | ⁎ | |
ND, Non-detectable; ⁎, sacrificed on week 4.
The weight of spleen of the antibody-injected animals was greater than that of the control. The mean weight of each spleen was 2.56 g for control macaques, 3.86 g for the animals injected with 0.5 mg/kg of antibody and 5.44 g for the animals injected with 5 mg/kg of antibody. However, 3 weeks after the last injection, the weight of injected animal spleens was not different from that of the control. The weight of other organs was in normal level (data not shown).
The organs were also examined for pathology. After the last injection, four animals from each group were sacrificed and organs from these animals were investigated. The spleens and the lymph nodes from the antibody-injected animals had significant pathomorphological changes, compared with control animals (Fig. 2A–H). In the “5 mg/kg group”, spleen displayed pathological changes correlated with the injection of equine F(ab')2 (Fig. 2B and C). There were mild lymph follicular hyperplasia and expanded germinal centers with proliferating follicular dendritic cells (FDC's) and macrophages. In the splenic cord, a mild to moderate plasma cell proliferation occurred. The pathological changes in lymph nodes were similar to those observed in spleens but the immune reaction was more overt (Fig. 2F and G). There was an apparent lymph follicular hyperplasia in cortical part and most of germinal center expanded. Macrophages proliferated, showed active phagocytosis and most of them were full of erythrocytes and hemosiderin. Other organs did not show significant pathological changes (data not shown).
Fig. 2.
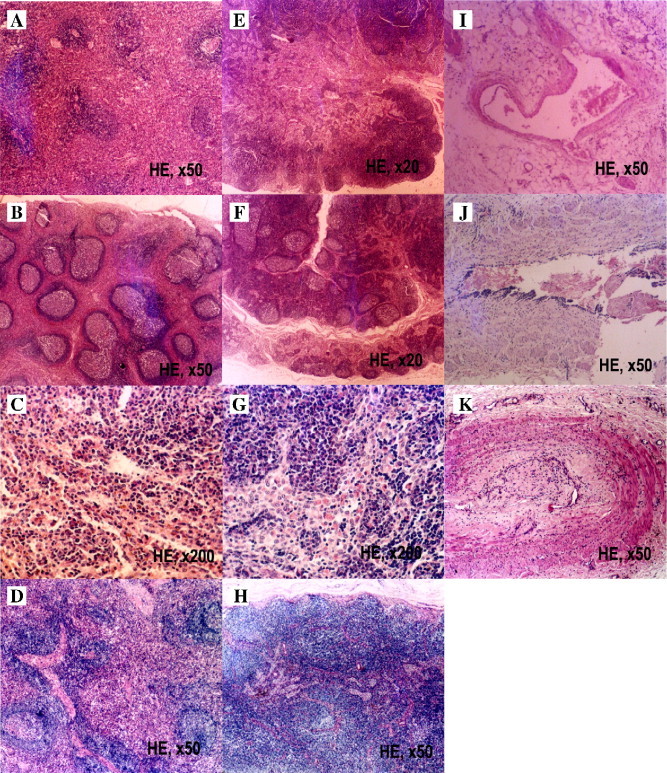
Immunostimulation of equine F(ab′)2 to macaque immune organs. The macaques received successive injections of 5 mg/kg body weight of equine anti-SARS–CoV F(ab′)2 everyday for 4 weeks with or without a 3-week recovery after the last injection. Panel A, the spleen of monkey injected with saline. Panels B and C, the spleen from a successively injected monkey. Panel D, the spleen from a monkey received successive injections but with a 3-week recovery after the last injection. Panel E, the lymph node of monkey injected with saline. Panels F and G, the lymph node from a successively injected animal. Panel H, the lymph node from a monkey that received successive injections with a 3-week recovery after the last injection. Panel I, the injection site of a saline-injected control macaque. Panel J, the injection site of a macaque that received successive injections. Panel K, the injection site of an animal injected successively but with a 3-week recovery after the last injection. The staining method and the magnification for each photo were indicated.
At the injection sites, 9 of 12 animals injected with equine anti-SARS–CoV F(ab′)2 showed some pathological changes in the dermis and subcutaneous tissue. The antibody-injected animals displayed partial angiolysis of vessel walls, proliferation and thickening of tunica intima, collagen fiber hyalinization, focal fibrinoid necrosis and some thrombosis in the lumen of blood vessels, compared to saline controls (Fig. 2I and J).
After a 3-week recovery from 4 weeks of successive injections, the immune reaction in the spleen and the lymph nodes resolved (Fig. 2D and H). Other organs looked normal, too. At the injection sites, some small vessels showed thrombus organization and there were many neutrophilic granulocytes in their walls (Fig. 2K).
The “0.5 mg/kg group,” showed pathological changes in the secondary lymphoid organs similar to those observed in the “5 mg/kg group,” but the animals displayed less plasma cell proliferation (data not shown).
4. Discussion
We have previously showed that equine anti-SARS–CoV F(ab′)2 prevents infection in cultured Vero E6 cells and in various animal models including the adult and the aged BALB/C mouse models [17], [18], the Golden Syria hamster and the Chinese hamster models [19], [20]. Furthermore, the antibody can provide a therapeutic protection to SARS–CoV-infected Vero E6 cells and in these animal models [17], [18], [19], [20].
However, the possibility that heterogeneous antibody might evoke a strong host immune response may inhibit its application in a clinical setting. Thus, development of human or humanized antibody against SARS–CoV is theoretically the ideal strategy to prevent infection, as it would be recognized as a ‘self’ component by human hosts and would not elicit an immune response in the host. For this reason and other considerations such as the difficulty of finding immune human donors and the risk related to the use of human blood products, human and humanized monoclonal antibodies against SARS–CoV components had been developed and have exhibited effectively preventive roles against SARS–CoV infection in vitro and in vivo [21], [22], [23], [24], [25]. Nevertheless, the major obstacle for the application of these mAbs in the clinic is the yield of mAb products. However, the heterologous antibodies, for example, equine IgGs, have an advantage in this respect. Furthermore, one theoretically potential advantage of the polyclonal IgGs is the broader antigenic coverage and the lower likelihood of emergence of escape mutants, although this theoretical advantage of the equine anti-SARS–CoV F(ab′)2 has not been studied here. In addition, the heterology of specific IgGs can be decreased through the preparation of F(ab′)2 fragments by cutting off the Fc fragment, thus F(ab′)2 should have higher specific neutralizing activity than complete IgG molecules, which means a smaller quantity of F(ab′)2 would be needed to neutralize the virus and this would reduce the possibility of an immune response against the F(ab′)2, although skin testing for hypersensitivity and possible desensitization [8] may be still necessary in practice.
In this study, we have investigated the pharmacology and the safety of the equine anti-SASR-CoV F(ab′)2 in macaques and rats. The assays for pharmacokinetics of equine anti-SARS–CoV F(ab′)2 in macaques and rats has verified the safety of using this type of antibody when administered into animals. Although the AUCs of the F(ab′)2 concentration are higher when higher amount of this agent is administered, the MRT (h), CL/F, VSS, t1/2 (h) and Kel have no difference between each dose of injections (1 mg/kg vs 3 mg/kg vs 10 mg/kg, respectively. Fig. 1 and Table 1). More importantly, when injected successively four times, all the pharmacokinetic parameters of the injected antibody are all the same between each injection time point (Fig. 1B and Table 2). The pharmokinetics data suggest that the F(ab′)2 fragments had the normal metabolism in injected animals.
We then investigate the tolerance and the immunity of hosts against the injected F(ab′)2 in macaques. All general physiological indexes have no significant difference in successively injected animals from the control, suggesting the good tolerance of the animals to this agent. However, we have really observed the local pathological changes in the injected sites and in the spleens and lymph nodes of the hosts. We have observed that 9 of 12 animals injected with equine anti-SARS–CoV F(ab′)2 show mild inflammatory response in the local sites of injection. The weight of spleens and lymph nodes from injected animals increased moderately compared with controls. The morphological observation indicate that there are moderate immune responses in the spleens and lymph nodes of the injected animals against the administered equine anti-SARS–CoV F(ab′)2 fragments. However, all the morphological changes in the secondary immune organs related with the injection of the F(ab′)2 disappeared after a 3-week recovery from the last injection. The morphological data indicate the hosts can tolerate the successive injection of the F(ab′)2. Corresponding with the morphological changes in lymph organs after successive injection of the F(ab′)2, the antibody against the injected equine F(ab′)2 can be detected in all the experimental animals at week 2 post-successive injection and even can be detected at week 7 (3-week recovery from the 4-week successive injection everyday). These data indicate that the heterogenous antibody can indeed elicit the immune response in inoculated hosts. Nevertheless, no experimental animals died of the antibody injection and the motivated host secondary lymphoid organs, the spleen and lymph nodes, recovered 3 weeks after the last injection, indicating the good tolerance of the host against this antibody injection. In addition, because the host anti-F(ab′)2 antibody appears 2 weeks after F(ab′)2 administration, there is enough time for the equine antibody to exert a protective effect at the early stage of SARS–CoV infection, as shown in our previous studies with this type of antibody in several animal models [17], [18], [19], [20], thus producing a marked effect during a large-scale SARS outbreak, or providing enough time for the combined application of other anti-SARS–CoV agents, such as antiviral drug, vaccines and so on.
Footnotes
All affiliated authors in this manuscript do not have a commercial or other association that might pose a conflict of interest. This work was supported by National Key Basic Research Program of China (“973” Projects): Fund for the Basic Research of SARS Prevention (2003CB514108), Key Research Project of Natural Science Foundation of China (No.30490240), Outstanding Youth Scientist Foundation of China (No. 30325020) and National Natural Science Foundation (No. 30571835 and 30500537).
Contributor Information
Bing Ni, Email: nibingxi@yahoo.com.
Yuzhang Wu, Email: wuyuzhang@yahoo.com.
References
- 1.Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R., St Claire M. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363(9427):2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci USA. 2004;101(17):6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci USA. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinatl J., Jr., Michaelis M., Hoever G., Preiser W., Doerr H.W. Development of antiviral therapy for severe acute respiratory syndrome. Antivir Res. 2005;66(2–3):81–97. doi: 10.1016/j.antiviral.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat Med. 2004;10(12 Suppl):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller M.A., Stiehm E.R. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev. 2000;13(4):602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt G., Kantipong P., Jongsakul K., de Souza M., Burnouf T. Passive transfer of scrub typhus plasma to patients with AIDS: a descriptive clinical study. QJM. 2001;94(11):599–607. doi: 10.1093/qjmed/94.11.599. [DOI] [PubMed] [Google Scholar]
- 10.Savoldo B., Goss J., Liu Z., Huls M.H., Doster S., Gee A.P. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72(6):1078–1086. doi: 10.1097/00007890-200109270-00017. [DOI] [PubMed] [Google Scholar]
- 11.Chiba T., Yokosuka O., Goto S., Fukai K., Imazeki F., Shishido H. Successful clearance of hepatitis B virus after allogeneic stem cell transplantation: beneficial combination of adoptive immunity transfer and lamivudine. Eur J Haematol. 2003;71(3):220–223. doi: 10.1034/j.1600-0609.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrantelli F., Rasmussen R.A., Hofmann-Lehmann R., Xu W., McClure H.M., Ruprecht R.M. Do not underestimate the power of antibodies—lessons from adoptive transfer of antibodies against HIV. Vaccine. 2002;20(Suppl 4):A61–A65. doi: 10.1016/s0264-410x(02)00389-4. [DOI] [PubMed] [Google Scholar]
- 13.Dahmen U., Dirsch O., Li J., Fiedle M., Lu M., Rispeter K. Adoptive transfer of immunity: a new strategy to interfere with severe hepatitis virus reinfection after woodchuck liver transplantation. Transplantation. 2004;77(7):965–972. doi: 10.1097/01.tp.0000113804.35096.8e. [DOI] [PubMed] [Google Scholar]
- 14.Soo YO, Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78(7):3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang J., Attanath P., Quiambao B., Singhasivanon V., Chanthavanich P., Montalban C. Evaluation of the safety, immunogenicity, and pharmacokinetic profile of a new, highly purified, heat-treated equine rabies immunoglobulin, administered either alone or in association with a purified, Vero-cell rabies vaccine. Acta Trop. 1998;70(3):317–333. doi: 10.1016/s0001-706x(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Ni B., Du X., Zhao G., Gao W., Shi X. Protection of mammalian cells from severe acute respiratory syndrome coronavirus infection by equine neutralizing antibody. Antivir Ther (Lond.) 2005;10(5):681–690. [PubMed] [Google Scholar]
- 18.Zhou L., Ni B., Luo D., Zhao G., Jia Z., Zhang L. Inhibition of infection caused by severe acute respiratory syndrome-associated coronavirus by equine neutralizing antibody in aged mice. Int Immunopharmacol. 2007;7(3):392–400. doi: 10.1016/j.intimp.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao G., Ni B., Jiang H., Luo D., Pacal M., Zhou L. Inhibition of severe acute respiratory syndrome-associated coronavirus infection by equine neutralizing antibody in golden Syrian hamsters. Viral Immunol. 2007;20(1):197–205. doi: 10.1089/vim.2006.0064. [DOI] [PubMed] [Google Scholar]
- 20.Luo D., Ni B., Zhao G., Jia Z., Zhou L., Pacal M. Protection from infection of SARS–CoV in a Chinese hamster model by equine neutralizing F(ab′)2. Viral Immunol. 2007;20(3) doi: 10.1089/vim.2007.0038. [DOI] [PubMed] [Google Scholar]
- 21.Duan J., Yan X., Guo X., Cao W., Han W., Qi C. A human SARS–CoV neutralizing antibody against epitope on S2 protein. Biochem Biophys Res Commun. 2005;333(1):186–193. doi: 10.1016/j.bbrc.2005.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J Virol. 2005;79(10):5900–5906. doi: 10.1128/JVI.79.10.5900-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci USA. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ter Meulen J., Bakker A.B., van den Brink E.N., Weverling G.J., Martina B.E., Haagmans B.L. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


