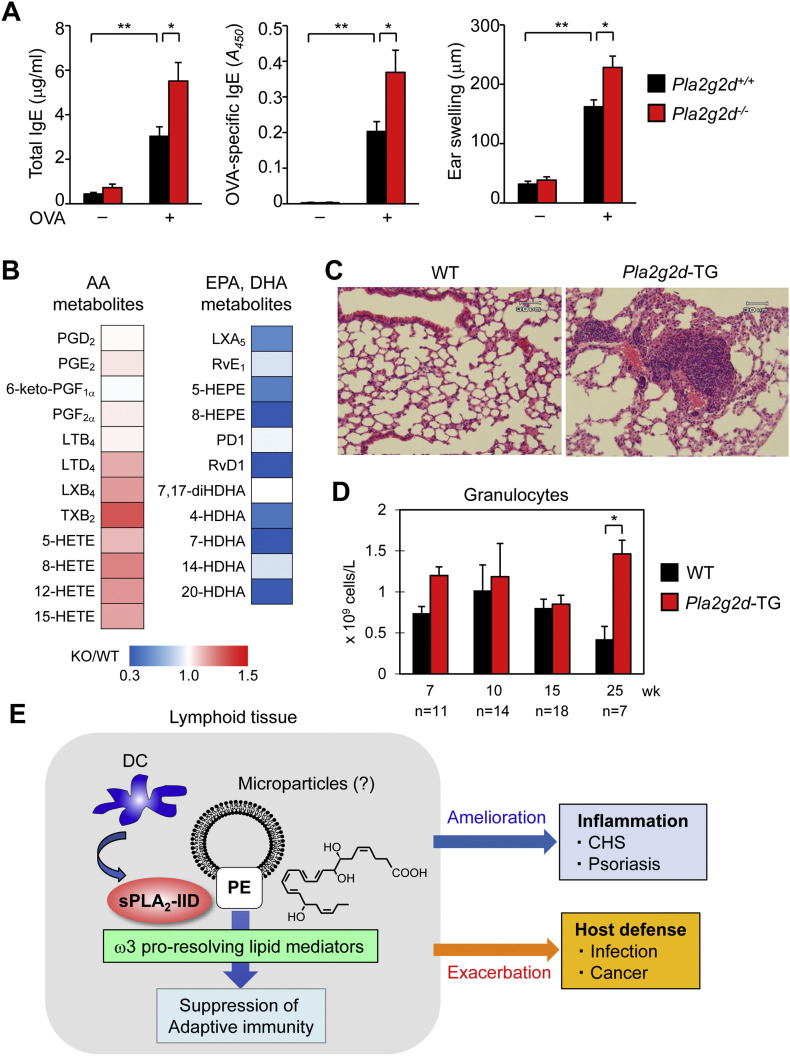
Fig. 1.
Properties and functions of sPLA2-IID. (A) Increased Th2 response in Pla2g2d−/− mice. Pla2g2d+/+ and Pla2g2d−/− mice (male, 8 weeks old) were immunized intraperitoneally on days 0, 7, and 14 with 10 μg of chicken OVA (Sigma-Aldrich) in 100 μl of saline mixed with 200 μl of alum (Alu Gel S, which contained 2% Al(OH)3; Serva). Seven days after the last immunization, the left and right ears of the mice were injected intradermally with 30 μg of OVA. Ear swelling was measured at 30 min after OVA challenge. Total and OVA-specific IgE levels in sera were measured by ELISA (Bethyl Laboratories). OVA-induced IgE levels and ear edema were elevated in Pla2g2d−/− mice relative to Pla2g2d+/+ mice (n = 5–7, mean ± SEM, *p < 0.05, **p < 0.01). (B) Lipidomic heat map profiling of ω6 AA- and ω3 EPA/DHA-derived lipid mediators in lymph nodes of Pla2g2d−/− (KO) mice relative to Pla2g2d+/+ (WT) mice. EPA/DHA-derived lipid mediators were decreased in KO mice [25,31]. The elevation of several AA metabolites in KO mice might have been due to increased lymph node inflammation. HETE, hydroxyeicosatetraenoic acid; HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; LX, lipoxin; PD, protectin; Rv, resolvin. (C) Lung histology of WT and Pla2g2d-TG mice (male, 34 weeks old). The lungs of Pla2g2d-TG mice had more pronounced leukocyte infiltration than those of WT mice. (D) Aged (25 weeks old), but not young (7–15 weeks old), Pla2g2d-TG mice had more circulating granulocytes than did age-matched WT mice (mean ± SEM, *p < 0.05). The results in (C, D) suggest that the increased immunosuppressive tone in the TG mice results in more opportunistic infection, and thereby airway inflammation. (E) A schematic diagram of sPLA2-IID action. In lymphoid tissues, sPLA2-IID is preferentially expressed in DCs and hydrolyzes PE in microparticles to provide ω3 EPA/DHA-derived pro-resolving lipid mediators (the structure of RvD1 is shown), which dampen adaptive immunity. As such, sPLA2-IID ameliorates Th1/Th17-dependent inflammation in CHS and psoriasis and perturbs host defense against infection and cancer.