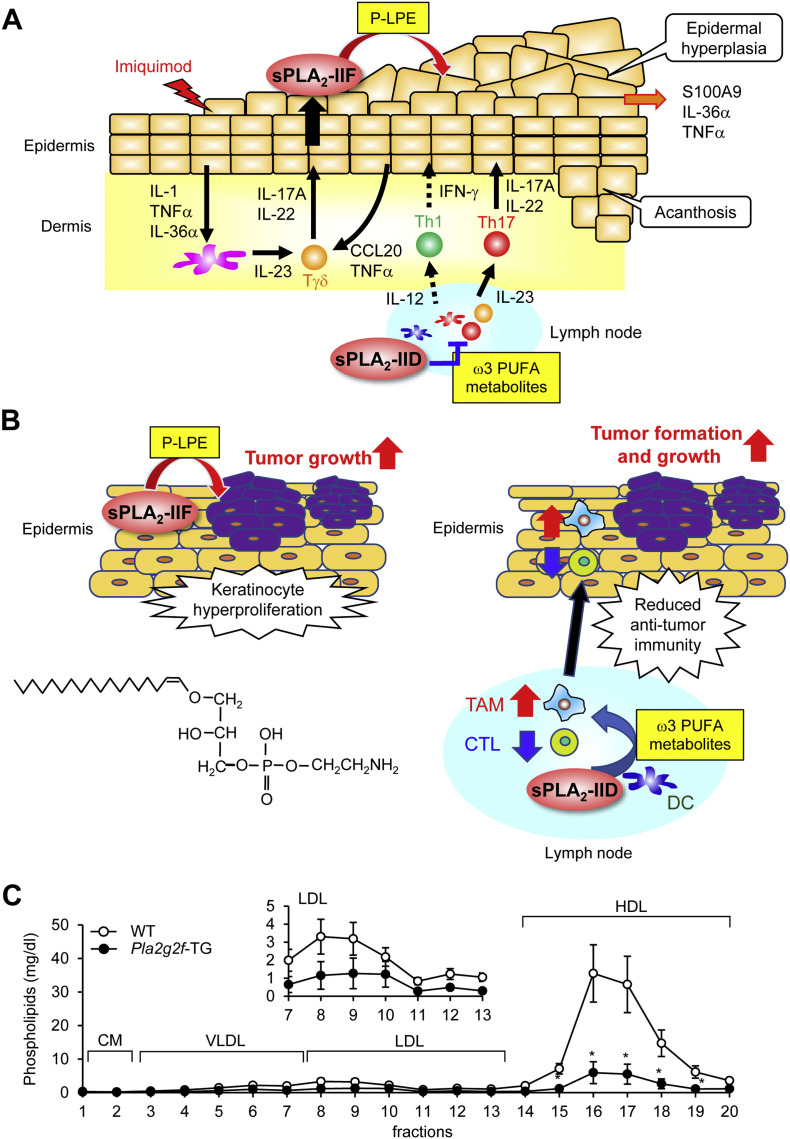
Fig. 3.
Properties and functions of sPLA2-IIF. (A, B) Distinct roles of sPLA2-IIF and sPLA2-IID in psoriasis and skin cancer [31,45]. (A) Following a psoriatic stimulus (imiquimod), sPLA2-IIF is induced in epidermal keratinocytes by Th17 cytokines derived from Tγδ and Th17 cells and hydrolyzes plasmalogen to give rise to lysoplasmalogen (P-LPE), which in turn promotes epidermal hyperplasia and inflammation. In contrast, sPLA2-IID blocks Th17 immunity in lymph nodes through production of ω3 PUFA metabolites, thereby putting a brake on psoriasis. (B) P-LPE produced by epidermal sPLA2-IIF promotes hypergrowth of skin cancer, without affecting its incidence. In contrast, ω3 PUFA metabolites produced by sPLA2-IID in lymph nodes decrease IFN-γ+CD8+ cytotoxic T cells (CTLs) and increase M2-like tumor-associated macrophages (TAMs), leading to reduced anti-tumor immunity. As such, sPLA2-IID facilitates tumor formation and growth. The structure of P-LPE is shown. (C) Lipoprotein profiles in Pla2g2f-TG and WT mice. The levels of phospholipids in HDL and LDL were markedly lower in Pla2g2f-TG mice than in WT mice (n = 4, mean ± SEM, *p < 0.05), suggesting that sPLA2-IIF, when overexpressed systematically, has the capacity to hydrolyze lipoprotein phospholipids in the circulation. LDL fractions are magnified in Inset. VLDL, very-low-density lipoprotein; HDL, high-density lipoprotein; CM, chylomicron.