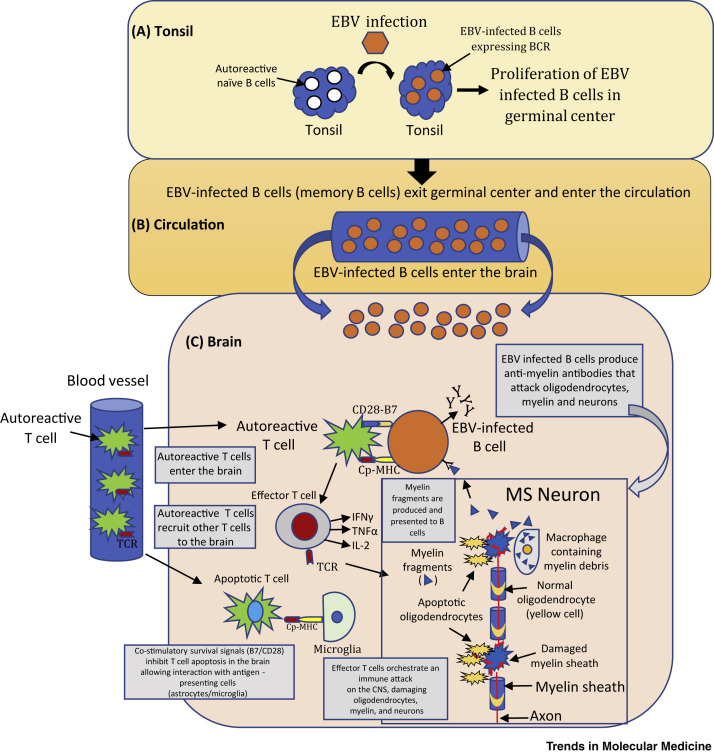
Figure 1.
The Autoreactive B Cell Hypothesis
(A) Tonsil to germinal center (GC): naïve B cells (white) are infected by Epstein–Barr Virus (EBV, orange) and proliferate in GCs. B cell receptor (BCR) and EBV proteins are expressed on latently infected autoreactive memory B cells. (B) Circulation: EBV-infected memory B cells exit the tonsil into the circulation (orange). EBV-specific cytotoxic CD8+ T cells normally control the number of EBV-infected B cells but, in the case of multiple sclerosis (MS), there is a defect in this mechanism. (C) EBV-infected B cells enter the MS brain residing for long periods of time where they produce oligoclonal IgG bands and pathogenic autoantibodies which attack and damage neurons. EBV-infected B cells provide co-stimulatory survival signals (B7) to CD28 receptors expressed on autoreactive T cells (green) that normally undergo apoptosis. Autoreactive T cells entering the brain are reactivated by EBV-infected B cells presenting CNS antigens (Cp) bound to MHC molecules. Autoreactive T cells produce cytokines [e.g., IL2, interferon (IFN)-γ, tumor necrosis factor (TNF)-β] and recruit other inflammatory cells (effector and cytotoxic T cells) that damage oligodendrocytes (yellow), myelin, and neurons. Adapted, with permission, from [18]. Abbreviations: B7, co-stimulatory molecule; CD28, T cell surface receptor; CNS, central nervous system; Cp–MHC, CNS peptides bound to MHC molecules; TCR, T cell receptor.