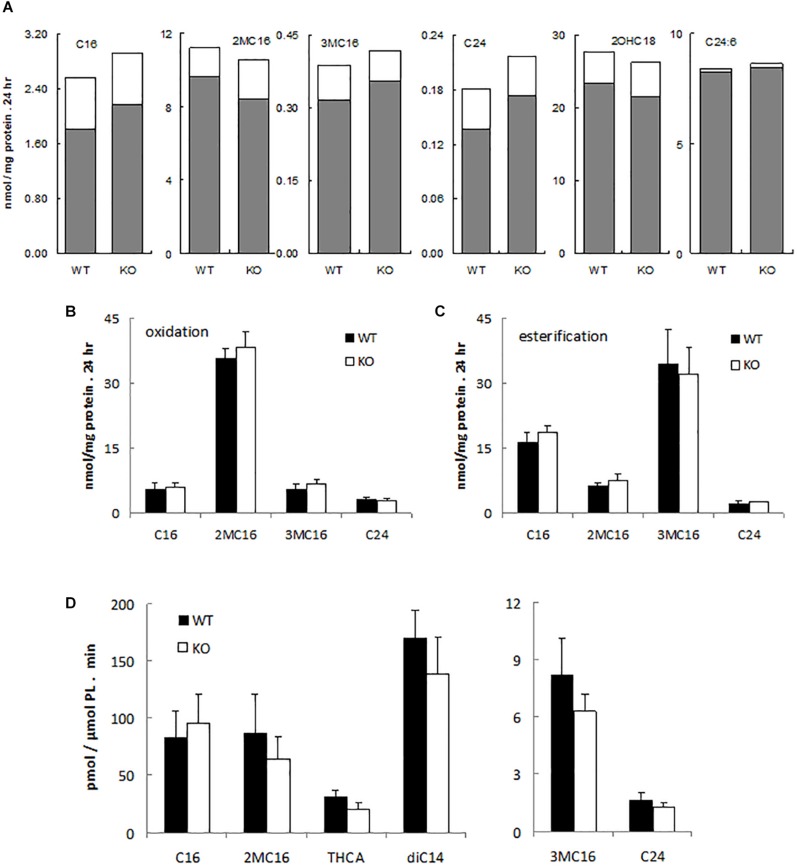
FIGURE 2.
α- and β-oxidation in PMP34 deficient cells. Primary MEF (A), immortalized MEF (B,C) or liver slices (D) from wild type (WT) and PMP34 knockout (KO) embryos or mice were analyzed for α- and β-oxidation using 14C-labeled substrates. Data for primary MEF (A), derived from a single WT or KO E14.5 embryo (C57BL/6 background), are based on single incubations at 310-430 μg protein/T25 falcon, followed by analysis of labeled oxidation products (white bars: CO2; gray bars, acid soluble material or formate in case of 3-methylbranched fatty acids), normalized to protein content of the monolayer. Incorporation of fatty acids in glycerolipids and total uptake was comparable between the genotypes (not shown). Similar findings were obtained when incubations were done at other cell densities. Data for immortalized fibroblasts (B,C), derived from 2 different embryos per genotype, are based on duplicate incubations at 110-180 μg protein/T25 falcon, and expressed as mean ± SD. Oxidation (B), normalized to protein, represent the sum of CO2 and acid soluble material or to CO2 plus formate in case of 3-methyl-branched fatty acids. Total uptake by fibroblasts, calculated as oxidation plus esterification, the latter representing the label recovered in cholesterylesters, triacylglycerols and phospholipids (C), was not different between WT and KO cells. Data for liver slices (D) are based on duplicate incubations with slices derived from 3 male animals per genotype and represent the sum of CO2 and acid soluble material or CO2 plus formate in case of 3-methyl-branched fatty acids, expressed as mean ± SD (normalized to phospholipids, PL). For no substrate, differences in CO2 or acid soluble material production were statistically significant except for THCA (*P < 0.05). Esterification rates for fatty acids were comparable (not shown). Dicarboxylic acids, 2-hydroxy long chain fatty acids, and bile acids are hardly or not incorporated into triacylglycerols and phospholipids, hence esterification of these substrates was not analyzed. C16, [1-14C]-hexadecanoic acid; 2MC16, 2-methyl-[1-14C]-hexadecanoic acid; 3MC16, 3-methyl-[1-14C]-hexadecanoic acid; C24, [1-14C]-tetracosanoic acid; 2OHC18, 2-hydroxy-[1-14C]-octadecanoic acid; C24:6, [2-14C]-6,9,12,15,18,21-tetracosahexaenoic acid; THCA, [26-14C]-3,7,12-trihydroxycholestanoic acid; diC14, [1,14-14C]-tetradecanedioic acid.