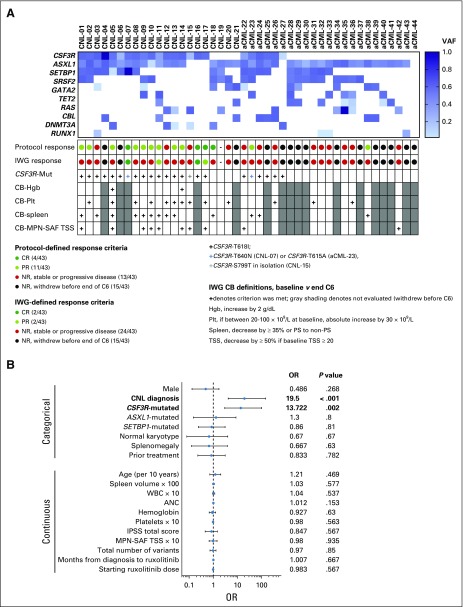
FIG 2.
(A) Summary figure of patients according to mutation profile, response assessment, and clinical benefit (CB). The heat map displays the 11 most commonly mutated genes in this study population according to variant allele frequency (VAF; darkest blue 100% and white 0%). The protocol-defined and International Working Group (IWG)–defined response assessments are color-coded by response. CSF3R-T618I mutation status is indicated as well as other pertinent CSF3R variants. CB was assessed by IWG criteria with one modification.16 We added the CB criterion of decrease in spleen volume by ≥ 35% (used in myelofibrosis pivotal studies).34 CB was not evaluated in patients who had a complete response (CR) by IWG criteria or in patients who withdrew before the end of cycle 6 (denoted by gray shading). (B) Forest plot for the unadjusted odds ratio (OR) and 95% CI obtained from univariate logistic regression models, with some continuous variables scaled in units of 10 or 100 to produce reasonable range of ORs. Data separated by categorical and continuous variables. Analyses on 43 evaluable patients were performed based on various demographic and disease characteristics and also starting doses of ruxolitinib. Boldface indicates variables that were found to be statistically significant. We also explored the adjusted effects, potential confounders, and effect modifiers using multivariable logistic regression models. Multivariable models were not presented because diagnosis is the single dominating risk factor for response, even after controlling for other important risk factors (ie, CSF3R mutation status and spleen volume). Although CSF3R mutation and spleen volume showed individual confounding effects, the estimated effect of diagnosis from a multivariable logistic regression model with both covariates included was very close to that from the univariate model. aCML, atypical chronic myeloid leukemia; ANC, absolute neutrophil count; C6, cycle 6; CNL, chronic neutrophilic leukemia; Hgb, hemoglobin; MPN-SAF, myeloproliferative neoplasm symptom assessment form; NR, no response; Plt, platelet; PR, partial response; PS, palpable spleen; TSS, total symptom score.