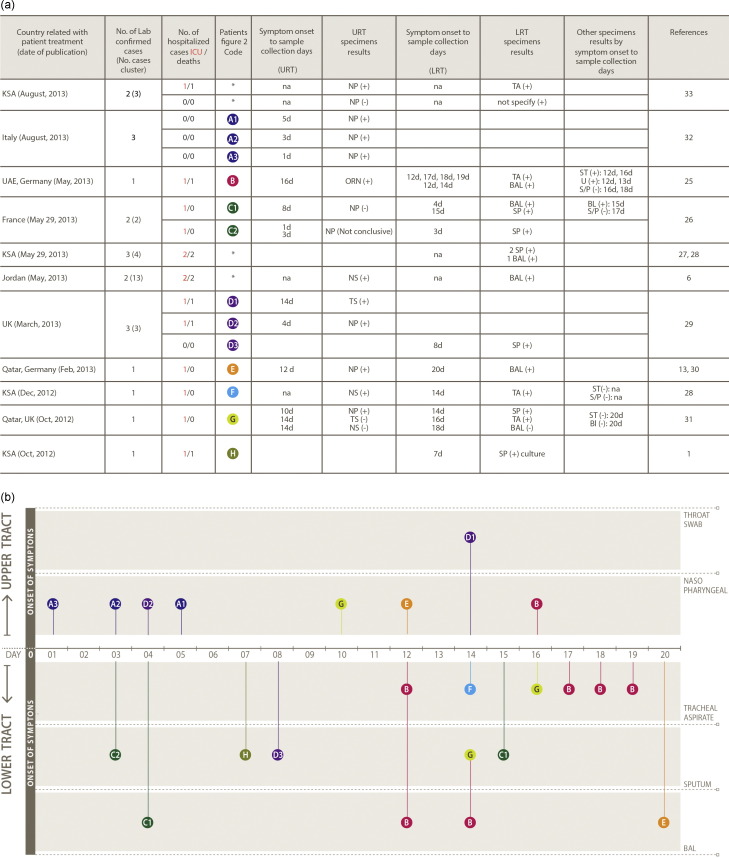
Fig. 2.
(A) Summary of case reports with diagnostic information relevant for laboratory preparedness. (naso-pharyngeal swab or aspirate (NP); throat swab (TS); nasal swab (NS); oro-nasal swab (ORS); tracheal aspirates (TA); Sputum (SP); bronchoalveolar lavage (BAL); Stool (ST);Urine (U); serum or plasma (S/P); positive (+); negative (−); upper respiratory tract (URT); lower respiratory tract (LRT); * not present in figure; not available data (na). (B) Summary of data from published literature and reports on MERS-CoV RT-PCR positive respiratory specimens by sample type, and timing of sampling since onset of symptoms (see Ref. [29]).