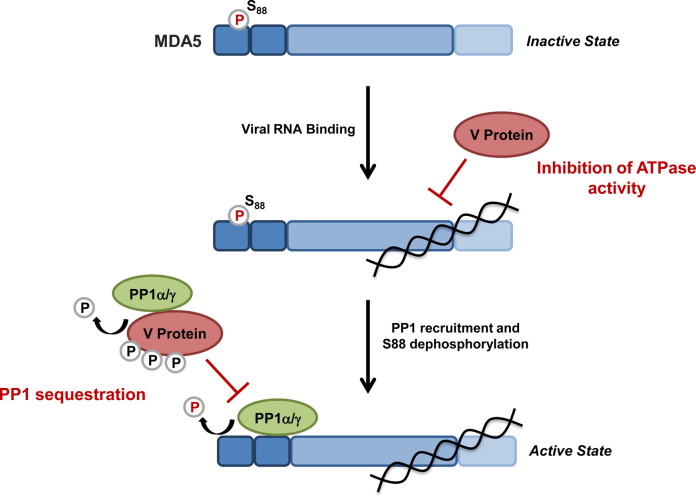
Fig. 6.
Suppression of MDA5 activity through paramyxoviral modulation of MDA5 phosphorylation and inhibition of its ATPase activity. The V proteins of measles virus (MV) and Nipah virus (NiV) sequester the phosphatases PP1-α/γ from MDA5, preventing the dephosphorylation of Ser88 in the N-terminal CARDs of MDA5. V protein-induced inhibition of MDA5 S88 dephosphorylation prevents MDA5-MAVS binding and downstream signaling. Furthermore, upon binding to PP1-α/γ, the V proteins serve as substrates for PP1-mediated dephosphorylation. The V proteins of several paramyxoviruses, including Parainfluenza virus type 5 (PIV5), bind directly to the helicase domain of MDA5, inducing conformational changes in MDA5, ultimately suppressing the ATP hydrolysis activity of MDA5 and thereby its filament formation on viral dsRNA.