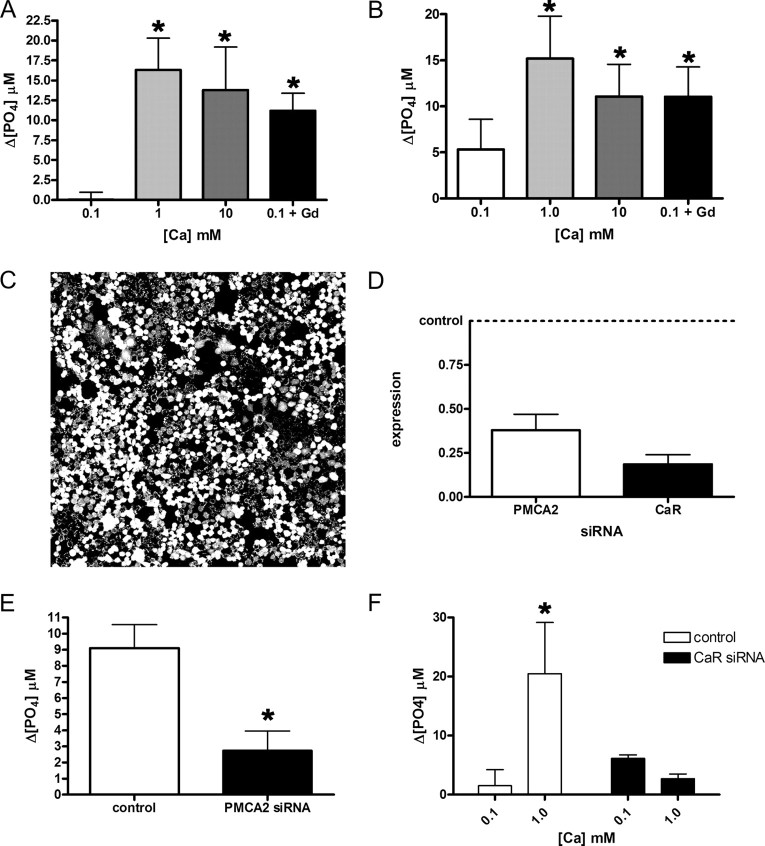
Fig. 7.
Activation of the CaR augments PMCA2 activity. Ca-ATPase activity in the plasma membrane fraction of mouse MECs (A) and EpH4 cells (B) increases when the concentration of calcium in the media is raised from 0.1 to 1 or 10 mm. Bars in A represent the mean of three experiments, whereas bars in B represent the mean of five experiments. In A, P < 0.05 for 0.1 mm calcium vs. 1 and 10 mm calcium; in B, P < 0.01 for 0.1 vs. 1 mm calcium and P < 0.05 for 0.1 calcium vs. 10 mm calcium. In addition, if 100 μm GdCl3, a CaR agonist, is added to these cells in media with 0.1 mm calcium, an increase in Ca-ATPase activity is also seen, compared with 0.1 mm calcium alone (P < 0.05 in A; P < 0.05 in B). C, We achieved high transfection efficiency as demonstrated with fluorescent oligonucleotides transfected into EpH4 cells. D, Transfection of siRNA for mouse PMCA2 reduced mRNA levels by almost 70%, whereas transfection of CaR siRNA reduced CaR mRNA by approximately 80%. E, Ca-ATPase activity in the plasma membrane fraction of EpH4 cells grown at 2 mm calcium was reduced by 75% after transfection with PMCA2 siRNA compared with transfection with the control siRNA (P < 0.05, three experiments). These data suggest that PMCA2 accounts for most of the Ca-dependent ATPase activity present in the plasma membrane of MECs. F, Ca-ATPase activity increased significantly when the extracellular calcium concentration was raised from 0.1 to 1 mm calcium in EpH4 cells transfected with control siRNA (P < 0.05, three experiments). However, there was no increase in Ca-ATPase activity in the plasma membrane fractions from cells transfected with CaR siRNA. These data suggest that the CaR mediates the response of PMCA2 to extracellular calcium. Statistically significant differences are denoted by asterisks.