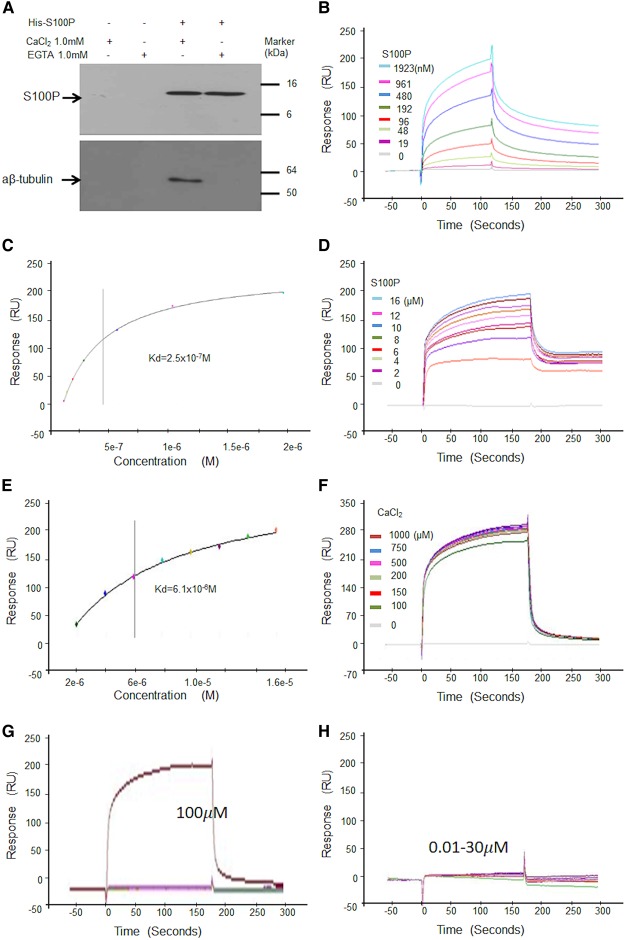
Figure 2. S100P binds to α,β-tubulins in vitro.
(A) Pulldown assay. His-S100P was loaded on His-binding beads. COS-7 cell lysates were applied to control beads (His-binding beads only) (a and b) or His-S100P beads (c and d) in the presence of 1 mM CaCl2 (a and c) or EGTA (b and d). After extensive washings, bound proteins were eluted with 300 mM imidazole from each bead and any His-S100P or α, β-tubulins were detected by Western blotting using anti-S100P (Upper panel) or anti-tubulin (Lower panel) antibodies. (B–F) Surface Plasmon Resonance (SPR) binding assays using α,β-tubulin immobilised on a chip and different concentrations of S100P in solution. (B) Typical binding curves of response units (RU) in arc seconds against time in physiological buffer of 0.15 M NaCl with 200 µM CaCl2 and multiple cycles of different concentrations of S100P. (C) A typical corresponding graph of response (RU) plotted against semilog of different concentrations of S100P for calculation of dissociation constant (Kd) at or near equilibrium for one experiment. (D) Typical binding curves of response (RU) in arc seconds in high salt buffer (0.5 M NaCl) with 200 µM CaCl2 and multiple cycles of different concentrations of S100P. (E) Corresponding graph of response (RU) plotted against semilog of different concentrations of S100P for calculation of Kd at or near equilibrium for one experiment. (F) Typical binding curves in physiological buffer of 0.15 M NaCl for fixed concentration of 2 µM S100P and multiple cycles of different concentrations of CaCl2. The values of the means ± SE from three experiments are quoted in Supplementary Table S2 (G and H). Typical binding curves of S100P (2 µM) to immobilised tubulin showing response units (RU) in arc seconds plotted against time for lower concentrations of CaCl2 in physiological buffer containing 0.15 M NaCl. The different concentrations of CaCl2 used were 0, 0.01, 0.03, 0.1, 0.3, 1.0, 3, 10, 30 and 100 µM. (G) shows that near maximal binding appeared when calcium ions reached 100 µM. (H) shows that no significant binding was detected when calcium ions were lower than 30 µM.