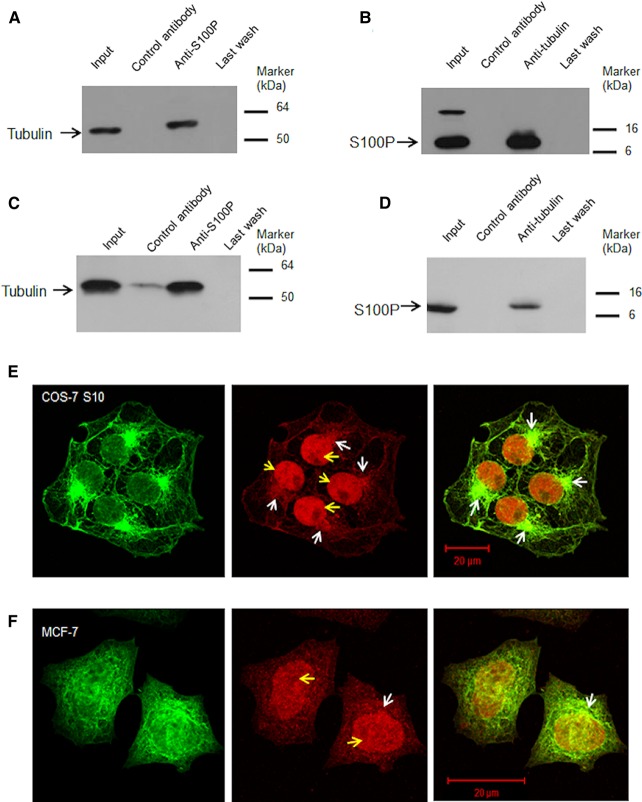
Figure 3. S100P binds to α,β-tubulins in vivo.
(A and B), Co-immunoprecipitations in S100P transfected cells. (A) S100P-induced COS-7 S10 cell lysates were divided in three parts, one part was Western blotted directly for tubulin (Input), remaining parts were combined and immunoprecipitated with anti-S100P serum bound to beads. The eluate from the control antibody-bound beads (Control antibody), eluate from anti-S100P-bound beads (Anti-S100P) and last wash from the anti-S100P-bound beads (Last wash) were probed with anti-tubulin serum on Western blots. (B) Similar lysates were divided into three parts, one part was Western blotted (Input) for S100P and remaining parts were combined and immunoprecipitated with anti-tubulin-bound to beads. The eluate from control antibody-bound beads (control antibody), from anti-tubulin-bound beads (Anti-tubulin) and last wash (Last wash) were probed with anti-S100P serum. (C and D) Co-immunoprecipitations of native S100P. Co-immunoprecipitations of S100 and tubulin in MCF-7 cell lysates using (C) anti-S100P or (D) anti-β-tubulin as well as control antibody were undertaken. The eluates and last washes from the antibody-bound beads were subject to Western blotting using (C) anti-β-tubulin or (D) anti-S100P as described above (Methods). Control antibody in (C) showed a weak band, which may be due to a cross-reacting contaminant. However, this does not affect the conclusion that complex formation can occur between endogenous S100P and tubulin. (E and F) Typical confocal images of (E) COS-7 S10 cells induced for 24 h with 1 µg/ml doxycycline or (F) MCF-7 cells and both immunofluorescently stained with TRITC-labelled anti-S100P (red) and FITC-labelled anti-tubulin antibodies (green). Yellow arrows indicate the nuclei and white arrows indicate the mitotic organising centres (MTOC). Bars = 20 µm.