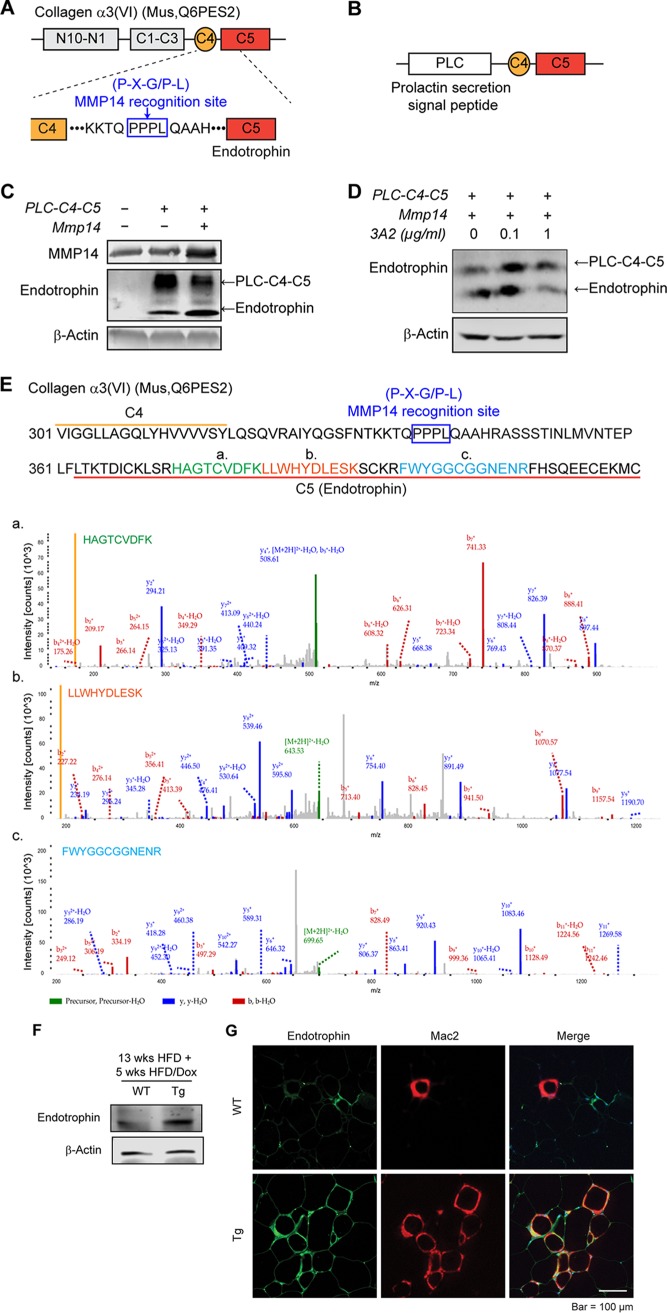
FIG 7.
MMP14 digests COL6 to produce endotrophin, which triggers fibrosis and inflammation in obese adipose tissue. (A) Schematic diagram showing the protein domains of mouse COL6α3. The MMP14 recognition site (P-X-G/P-L) between C4 and C5 domains is indicated within the blue box. (B) Schematic diagram showing the structure of pcDNA3.1-Plc-C4-C5 plasmid. The prolactin secretion signal peptide (Plc) was fused at the N terminus of the plasmid. (C) Western blotting of MMP14 and endotrophin in the 293T cells transfected with pcDNA3.1, Plc-C4-C5, or Plc-C4-C5, together with MMP14. β-Actin was used as loading control (representative of three experiments is shown). (D) Western blotting of endotrophin in 293T cells. The cells were cotransfected with Plc-C4-C5 and MMP14 and then treated with MMP14 monoclonal antibody 3A2 at the indicated doses. β-Actin was used as a loading control (representative of three trials). (E) Amino acid sequence of the C4-C5 terminal of COL6α3. Three peptides (indicated in green [a], orange [b], and blue [c]) which match parts of the sequence of C5 (endotrophin) were detected with high fidelity in the eWAT of HFD fed mice by mass spectrometry (LC-MS/MS). The MS spectra of three peptides were shown at the bottom. (F) Western blotting of endotrophin in the eWAT of MMP14 Tg and WT mice after long-term HFD feeding. The endotrophin was enriched from samples by immunoprecipitation with antiendotrophin antibody (n = 5 per group, representative of three trials). (G) Co-IF staining of endotrophin and Mac2 (marker of macrophage) in the eWAT of MMP14 Tg and WT mice after long-term HFD feeding. The right panel shows the colocalization of endotrophin and macrophages (indicated in yellow) (n = 5 per group, representative of three trials).