Abstract
Purpose
Gastric cancer (GC) is a malignant disease of digestive tract. Clinically, radiation therapy is widely applied in treating GC, while with undesirable outcome due to tumor re-proliferation and recurrence and metastasis after radiation. Therefore, it is crucial to explore potential molecular mechanisms to develop therapeutic strategies. The present study found that miR-26a-5p has low expression in GC patients and could regulate Wnt5a to inhibit tumor growth, which was a potential therapeutic target for GC. To explore the expression and related mechanism of miR-26a-5p and Wnt5a in GC.
Patients and Methods
MiR-26a-5p and Wnt5a were extracted from the transcriptome data of GC downloaded from TCGA database for analysis. The expression levels of miR-26a-5p and Wnt5a in patients’ tissues and serum were detected by qRT-PCR, and their correlation with patients’ pathological data and survival was analyzed. In addition, miR-26a-5p and Wnt5a overexpression and inhibition vectors were transfected into cells to observe the effects on the proliferation, invasion and apoptosis of GC cells. The relationship between miR-26a-5p and Wnt5a was analyzed by dual luciferase report.
Results
The database and clinical samples showed that miR-26a-5p level was low while Wnt5a was high in GC. MiR-26a-5p level decreased in patients with stage III+IV, lymphatic metastasis and tumor ≥3cm, and Wnt5a was contrary to that of the miR-26a-5p, with diagnostic value. Overexpressed miR-26a-5p and inhibited Wnt5a enhanced apoptosis, decreased proliferation and invasion, reduced Bcl-2 and β-catenin proteins, and elevated Caspase 3, E-cadherin and Bax proteins, while inhibited miR-26a-5p and over-expressed Wnt5a showed the opposite results. Dual luciferase report confirmed that miR-26a-5p targeted to regulate Wnt5a, and rescue experiments found that these effects could be counteracted by reducing miR-26a-5p level.
Conclusion
Overexpressed miR-26a-5p can inhibit Wnt5a expression, promote cell apoptosis, and suppress cell proliferation and invasion in GC.
Keywords: TCGA, miR-26a-5p, Wnt5a, gastric cancer, survival, diagnosis
Introduction
Along with the changes in people’s diet structure, the incidence of digestive system diseases is on the rise.1 As one of the most common digestive system tumors, gastric cancer (GC) has been reported to be responsible for 800,000 deaths among 1 million new GC patients found worldwide, with tremendous hazards on which patients’ health. Therefore, it is high time to find an effective treatment to improve the prognosis of patients.2,3 Currently, surgical approaches appear to be the most effective clinical treatment for GC. However, as it shows little symptoms at the early stage, a majority of patients are only to be found in advanced stages, missing the best time for treatment, some even presenting symptoms like metastasis and infiltration, which increases the difficulty of treatment.4,5 Therefore, it is urgent to elucidate the relevant mechanisms of GC and find potential prognostic targets, aiming to enhance the prognosis of GC patients.
MicroRNA (miR) is a highly conserved endogenous short non-coding RNA with a length of about 21-25nt.6 Previous studies have shown that miR-mediated mRNA degradation or mRNA translation is inhibited by complementary negative regulatory protein expression between miR-regulated and target genes in the 3ʹ-untranslated region (3ʹUTR).7,8 As a family of important regulatory factors, miR plays an important part in various biological pathways of the body like apoptosis, proliferation, differentiation and metabolism.9,10 In previous studies,11–13 it was revealed that miR is differentially expressed in lung cancer, liver cancer, breast cancer, esophageal cancer and other cancers. As a member of the miR family, miR-26a-5p (also known as miR-26a) has been used as a tumor suppressor gene in previous studies14 and has low expression in a variety of tumors. Ghanbari et al14 found that miR-26a-5p is reduced in colon cancer, whereas in Qiu’s et al15 study, miR-26a is indicated as a potential diagnostic marker for GC, whose mechanism remains the subject of investigation. Wnt5a belongs to the WNT family. Previous studies have found that16 Wnt5a is closely related to the metastasis of various malignant tumors, and as a promoter of non-classical Wnt signaling pathway, it can antagonize the WNT signaling pathway. Studies have found that17–19 Wnt5a is overexpressed in various tumors such as primary GC, non-small cell lung cancer and glioma. Here through the prediction of miR-target genes, it was found that there was a possible target binding site between miR-26a-5p and Wnt5a, but the specific mechanism between the two is still unclear.
Therefore, this study was set out to analyze the role of miR-26a-5p and Wnt5a in GC, providing potential therapeutic targets for clinical data.
Materials and Methods
Main Reagents, Instruments and Cells
Cell Source
Human normal gastric epithelial cell lines CSN, GC cell lines SNU-1, SNU-16, NCI-N87, AGS were purchased from American ATCC: The Global Bioresource Center, with the item number of CRL-6246, CRL-5971, CRL-5974, CRL-5822, CRL-1739, respectively. SGC-7901 were obtained from Shanghai Institutes for Biological Sciences, Chinese academy of sciences, with the item number of CBP60500.
Main Reagents and Instruments
CCK-8 kit was purchased from Shanghai Beyotime Biotechnology Co., Ltd., with item number: C0037. Transwell kit, DMEM, PBS, bovine fetal serum, Penicillin-Streptomycin were purchased from Gibco, USA, with item number of 1142802, 10566024, 10010023, 26400044, 15070063, respectively. RIPA kit, BCA protein kit, ECL luminescence kit, trypsin, Lipofectamine™ 2000 Transfection Reagent were all obtained from Thermo Scientific Company, USA, with the corresponding item number of 89900, 23250, 32209, 90059, 11668019. Wnt5a, Caspase 3, Bcl-2, Bax, E-cadherin, β-catenin, β-actin, HRP-labeled goat anti-mouse IgG II antibody were purchased from R&D Company, with the corresponding item number of cab174963, ab13847, ab182858, ab32503, ab1416, ab2365, ab MAB8929, HAF007. TransScript Green miRNA Two-Step qRT-PCR SuperMix and TransScript II Green Two-Step qRT-PCR SuperMix were obtained from Beijing TransGen Biotech, with the item number of AQ202-01, AQ301-01, respectively. Annexin V/PI Apoptosis Detection Kit was purchased from Shanghai Yeasen Biotechnology Co., Ltd., with item number: 402ES20. Dual luciferase reporter gene assay kit was purchased from Beijing Solarbio Technology Co., Ltd., with item number: D0010 PCR (ABI, 7500), flow cytometry from BD Company, FACS Canto II, and Microplate Reader from BioTek Company, Perkin Elmer, USA. All primers were synthesized by Shanghai GenePharma Co., LTD.
TCGA Data Analysis
Accessed TCGA database (https://portal.gdc.cancer.gov/), selected the Repository into data browse and download page, downloaded the stomach high-throughput sequencing of RNA, miR samples and their corresponding Metadata and the Manifest and Cart files, among which RNA specimens of 373 cases of GC were obtained, including cancer sample of 343 cases and adjacent cancer samples of 30 cases. There were 452 cases of miR-GC samples, including 410 cancer samples and 42 adjacent cancer samples. The matrix file was synthesized using the scripts of RNA_merge.pl and microRNA_merge.pl, and the ID was converted by using the scripts of ensemblToSymbol_incRNA.pl. The expression levels of miR-26a-5p and Wnt5a in all patients were extracted and converted to log (X+ 1,2) for analysis.
Sample Collection
A total of 35 patients with GC treated in First Affiliated Hospital, Heilongjiang University of Chinese Medicine from May 2015 to March 2018 were enrolled as the study group, including 22 male and 13 female with an average age of 61.6±5.0 years. In addition, another 35 healthy subjects were assigned to the control group, which include 25 male and 10 female, with an average age of 60.5±4.2 years. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital, Heilongjiang University of Chinese Medicine. No significant difference was observed as regard to age and gender between the two groups (P>0.05). The patient’s tissues were collected during the operation, while some tissues were collected during pathological biopsy of patients who could not be operated, and serum was collected from patients in the patient group and the control group. The inclusion criteria were as follows: Patients aged over 18 years old, who were diagnosed as GC by pathological examination, and met the TNM staging criteria issued by AJCC in 2017.20 The patients and their families were informed and signed the informed consent, and the clinical data of the patients were complete. In contrast, the exclusion criteria were as follows: Patients associated with other malignant tumors, patients with liver and kidney disease, patients with infection before admission, patients without previous treatment history (surgery, chemotherapy, radiotherapy or antibiotic treatment) before this study, and patients with an estimated survival of less than 3 months. Patients were followed up for their survival status over a period of 5 years through telephone and outpatient review, which was conducted every 3 months in the first year and every 6 months from the second to the fifth year. This study is in line with the Declaration of Helsinki.
Cell Culture and Transfection
Human GC cell lines SNU-1, SNU-16, NCI-N87, AGS, SGC-7901 and normal human gastric epithelial cell lines CSN have all been tested and identified by cell bank. The purchased cells were adjusted to 1*105 cells/mL and transferred to DMEM medium (10% bovine fetal serum, penicillin streptomycin double antibody) and cultured in a humidified incubator containing 5% CO2 at 37°C. MiR-26a-5p-mimics (overexpressed sequence), miR-26a-5p-inhibitor (inhibition sequence), miR-NC (negative control), sh-Wnt5a (targeted overexpression), si-Wnt5a (targeted inhibition sequence), si-NC (negative control RNA) were established, and the Lipofectamine™ 2000 kit was used to transfect the cells. All procedures were performed strictly according to the corresponding kit’s instructions.
QRT PCR Detection
Peripheral blood of 5mL was collected from the control group and the patient group before operation, and the serum was set aside for 30min and then centrifuged at 3000rpm for 10min. Next, the collected tissues, serum and cells were extracted by TRIzol kit, whose total RNA purity, concentration and integrity were detected by UV spectrophotometer and agarose gel electrophoresis. miR-195-5p and YAP reverse transcription were performed strictly according to the kit instructions. The amplification system of miR-195-5p was as follows: cDNA: 1um, upstream and downstream primers: 0.4μL respectively, 2×TransTaq® Tip Green qPCR SuperMix:10μL, Passive Reference Dye (50X): 0.4μL, and ddH2O was added to complete to 20μL. The amplification conditions were as follows: PCR reaction conditions: Pre-denaturation at 94°C for 30s, denaturation at 94°C for 5s, annealing at 60°C for 30s, totalling for 40 cycles. The YAP amplification system: cDNA: 1μL, upstream and downstream primers: 0.4μL respectively, 2X TransScript® Tip Green qPCR SuperMix: 10μL, Passive Reference Dye (50X): 0.4μL, and Nuclease-free Water completion up to 20um. Amplification conditions: Pre-denaturation at 94°C for 30s, denaturation at 94°C for 5s, annealing at 60°C for 30s, and a total of 40 cycles were carried out. Three repeat holes were set for each sample, and the experiment was carried out for a total of 3 times. U6 was used as the internal reference for miR, GAPDH as the internal reference for mRNA, and 2−ΔΔct was employed to analyze the data.21 Primer sequences are shown in Table 1.
Table 1.
Primer Sequences
| Gene | Upstream Primers | Downstream Primers |
|---|---|---|
| miR-26a-5p | 5ʹ-ACACTCCAGCTGGGTTCAAGTAATCCAGGA-3ʹ | 5ʹ-TGGTGTCGTGGAGTCG-3ʹ |
| Wnt5a | 5ʹ-ATTCTTGGTGGTCGCTAGGTA-3ʹ | 5ʹ-CGCCTTCTCCGATGTACTGC-3ʹ |
| U6 | 5ʹ-CTCGCTTCGGCAGCACA-3ʹ | 5ʹ-ACGCTTCACGAATTTGCGT-3ʹ |
| GAPDH | 5ʹ-CGGAGTCAACGGATTTGGTCGTATTGG-3ʹ | 5ʹ-GCTCCTGGAAGATGGTGATGGGATTTCC-3ʹ |
WB Detection
The total protein was extracted from the cultured cells by RIPA lysis, and the protein concentration was determined by BCA method. Then protein concentration was adjusted to 4μg/μL, electrophoretically separated by 12% SDS-PAGE before transferring to PVDF membrane. The cells were stained with Ponceau S working solution, washed with PBST for 5 mins, sealed with 5% skimmed milk powder for 2 hrs. Next, Wnt5a. Caspase 3, Bcl-2, Bax, E-cadherin, β-catenin I antibody (1:1000) were added and sealed overnight at 4°C. The first antibody was removed by washing the membrane, followed by horseradish peroxidase labeled goat anti-rabbit secondary antibody (1:500), incubated at 37°C for 1h, and rinsed with PBS for 3 times, 5min each. Developed in a dark room, dried the excess liquid on the membrane with filter paper, and developed with ECL luminescence. Finally, the protein bands were scanned and the gray values were analyzed by Quantity One software. The relative expression level of the protein was equal to the gray value of the target protein band/the gray value of the β-Actin protein band.
Cell Proliferation Assay (CCK-8)
Cells were collected 24 hrs after transfection, adjusted to 4*106 cells, inoculated on 96-hole plates, and cultured for 0h, 24h, 48h and 72h. Next, each hole was incubated at a temperature of 37°C for 2 hrs with 10μL CCK solution and 90μL Dulbecco’s modified eagle medium (DMEM). Then, the OD value of each group was measured at 450-nm absorbance by Microplate Reader.
Cell Invasion Detection (Transwell)
Cells were collected 24 hrs after transfection, adjusted to 5*104 cells, and inoculated on 6-hole plates. Next, the cells were washed twice with PBS and were inoculated in the upper chamber, 200μL DMEM culture medium was added in the upper chamber, 500μL DMEM (containing 20%FBS) was added in the lower chamber, and cultured at a temperature of 37°C for 48 hrs. The stroma and cells that did not cross the membrane surface were wiped and washed with PBS for 3 times, followed by a 10 mins’ fixing with paraformaldehyde, and then washed with double steamed water for 3 times. After drying, they were stained with 0.5% Crystal Violet Stain Solution, and the cell invasion was observed using a microscope.
Cell Apoptosis Detection (Flow Cytometry)
The transfected cells were washed twice with PBS after digestion with 0.25% trypsin, added with a binding buffer of 100 mL, and then disposed into 1*106/mL suspension. Added Annexin V-FITC and PI in turn, incubated at room temperature for 5 mins before detected by FC500MCL flow cytometry system. The experiment was repeated three times to get the average value.
Target Gene Detection
Downstream target genes of miR-26a-5p were predicted by Targetscan7.2. The wild-type (Wt) and mutant (Mut) of pmirGLO-Wnt5a-3ʹUTR, as well as the mutants of miR-26a-5p-mimics and microRNA-NC were transfected into HEK293T cells by Lipofectamine 2000 kit. Luciferase reporter assay showed that pmirGLO-Wnt5a-3ʹUT Wt and pmirGLO-Wnt5a-3ʹUTR Mut, miR-26a-5p-mimics and mir-NC co-transfected SGC-7901 and NCI-N87 cells. Luciferase activity was determined using a dual luciferase reporter gene assay kit.
Statistical Analysis
In this study, the collected data were statistically analyzed using SPSS20.0 software, and plotted by GraphPad 7. K–S test was used to analyze the distribution of dose data, among which the normal distribution data were expressed by mean ± standard deviation (Means±SD). An independent sample t test was adopted for comparison between groups. Data that did not conform to the normal distribution were expressed by quartile [Meas(P25~P75)], analyzed by nonparametric test, and expressed by Z. Multigroup comparisons were analyzed by one-way ANOVA and expressed as F. LSD-t test was used for pairwise comparison after the event, and repeated measurement ANOVA was used for multiple time points, represented by F. Bonferroni was used for post-test and ROC was adopted to map the diagnostic value of miR-26a-5p and Wnt5a in GC. Pearson test was used to analyze the relationship between the expression of miR-26a-5p and Wnt5a in serum and tissues of patients. The 5-year survival of the patients was plotted by K-M survival curve and analyzed by Log-rank test. Cox regression analysis was used to determine the independent factors affecting prognosis of GC patients. P<0.05 was supposed to be statistically different.
Results
The Expression of miR-26a-5p and Wnt5a in TCGA Database
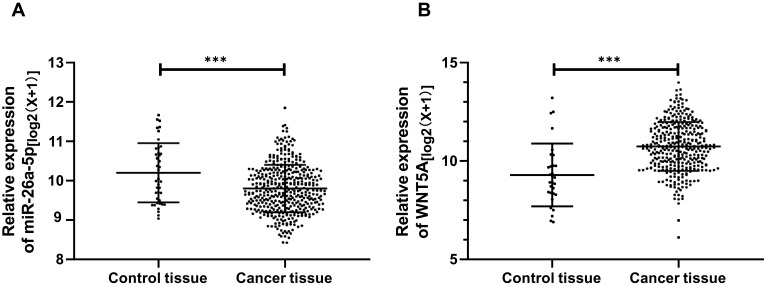
The expression of miR-26a-5p and Wnt5a in TCGA database was extracted and analyzed. It showed that the miR-26a-5p expression in cancer tissues was significantly lower than that of the control tissues (P<0.001), and the comparison of Wnt5a indicated that the Wnt5a expression in cancer tissues was significantly higher than that of the control tissues (P<0.001) (Figure 1).
Figure 1.
The expression of miR-26a-5p and Wnt5a in TCGA database. (A) MiR-26a-5p expression in hepatocellular carcinoma; (B) Wnt5a expression in hepatocellular carcinoma. ***Represented P<0.001.
The Clinical Value of miR-26a-5p and Wnt5a in Patients with GC
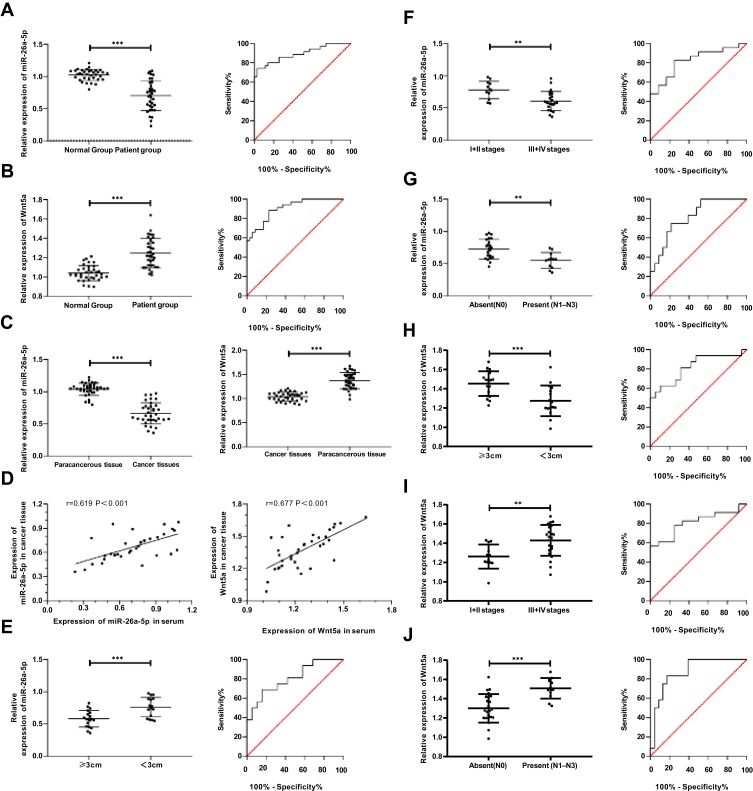
By detecting the expression of miR-26a-5p and Wnt5a in serum of patients and normal group, it was found that the miR-26a-5p expression in patient group was significantly lower than that of the normal group, while the expression of Wnt5a was significantly higher (P<0.001). The expression of miR-26a-5p and Wnt5a in cancer tissues and adjacent tissues were compared and the results were consistent with those in serum (P<0.001). Pearson correlation analysis showed that the expression of miR-26a-5p and Wnt5a in patients’ tissues and serum was positively correlated (P<0.05). In addition, judged by the median value of the expression, miR-26a-5p and Wnt5a were divided into corresponding high and low expression groups. By analyzing the relationship between miR-26a-5p, Wnt5a and the pathological data of patients, it was found that patients with low expression of miR-26a-5p and patients with high expression of Wnt5a had higher probability of stage III+IV, lymph node metastasis and tumor ≥3cm than their corresponding groups. Furthermore, the expression of miR-26a-5p and Wnt5a in different TNM stages, lymphatic metastasis and tumor size were compared and found to be different. The ROC curve analysis revealed that miR-26a-5p and Wnt5a had certain diagnostic value in TNM stage, lymphatic metastasis and tumor size, and Multivariate analysis found that TNM staging was an independent factor affecting the prognosis of patients as shown in Figure 2, Tables 2–4.
Table 3.
ROC Indicators
| Indicators | AUC | Std | 95% CI | P value | Specificity | Sensitivity | Yoden Index | Cut-Off |
|---|---|---|---|---|---|---|---|---|
| miR-26a-5p | 0.892 | 0.039 | 0.815–0.969 | <0.001 | 97.14% | 74.29% | 71.43% | <0.865 |
| Wnt5a | 0.904 | 0.034 | 0.837–0.970 | <0.001 | 77.14% | 88.57% | 65.71% | >1.094 |
| miR-26a-5p in tumor size | 0.806 | 0.074 | 0.661–0.951 | 0.002 | 84.21% | 68.75% | 52.96% | >0.716 |
| miR-26a-5p in TNM staging | 0.823 | 0.071 | 0.683–0.962 | 0.002 | 75.00% | 82.61% | 57.61% | <0.725 |
| miR-26a-5p in Lymphatic metastasis | 0.815 | 0.072 | 0.674–0.957 | 0.003 | 78.26% | 75.00% | 53.26% | <0.579 |
| Wnt5a in tumor size | 0.819 | 0.075 | 0.672–0.966 | 0.001 | 89.47% | 62.50% | 51.97% | <1.282 |
| Wnt5a in TNM staging | 0.808 | 0.073 | 0.665–0.951 | 0.003 | 100.00% | 56.52% | 56.52% | >1.441 |
| Wnt5a in Lymphatic metastasis | 0.873 | 0.060 | 0.756–0.991 | <0.001 | 82.61% | 83.33% | 65.94% | >1.427 |
Figure 2.
The expression and clinical value of miR-26a-5p and Wnt5a in gastric cancer. (A) Expression and diagnostic value of miR-26a-5p in serum; (B) Expression and diagnostic value of Wnt5a in serum; (C) Expression of miR-26a-5p and Wnt5a in tissues; (D) Correlation of miR-26a-5p and Wnt5a in tissue and serum; (E) Expression and diagnostic value of miR-26a-5p in differentiating tumor size from ≥3 cm to <3 cm; (F) Expression and diagnostic value of miR-26a-5p in differentiating I+II from III+IV patients; (G) Expression and diagnostic value of miR-26a-5p in differentiating lymphatic metastasis; (H) Expression and diagnostic value of Wnt5a in differentiating the size of tumors (> 3cm) from < 3cm; (I) Expression and diagnostic value of Wnt5a in differentiating stage I+II from stage III+IV patients; (J) Wnt5a expression and diagnostic value in differentiating lymphatic metastasis patients. **Represented P<0.01, and ***Represented P<0.001.
Table 2.
Relationship Between miR-26a-5p, Wnt5a and Pathological Data of Gastric Cancer Patients
| Indicators | miR-26a-5p | P value | Wnt5a | P value | |||
|---|---|---|---|---|---|---|---|
| High Expression (n=17) | Low Expression (n=18) | High Expression (n=17) | Low Expression (n=18) | ||||
| Gender | 0.358 | 0.238 | |||||
| Male (n=22) | 12 (70.59) | 10 (55.56) | 9 (52.94) | 13 (72.22) | |||
| Female (n=13) | 5 (29.41) | 8 (44.44) | 8 (47.06) | 5 (27.78) | |||
| Age (years) | 0.105 | 0.826 | |||||
| ≥60 (n=22) | 13 (76.47) | 9 (50.00) | 11 (64.71) | 11 (61.11) | |||
| <60 (n=13) | 4 (23.53) | 9 (50.00) | 6 (35.29) | 7 (38.89) | |||
| Tumor size | 0.028 | 0.011 | |||||
| ≥3cm (n=19) | 6 (35.29) | 13 (72.22) | 13 (76.47) | 6 (33.33) | |||
| <3cm (n=16) | 11 (64.71) | 5 (27.78) | 4 (23.53) | 12 (66.67) | |||
| Degree of differentiation | 0.227 | 0.632 | |||||
| Medium-high differentiation (n=11) | 7 (41.18) | 4 (22.22) | 6 (35.29) | 5 (27.78) | |||
| Low differentiation (n=24) | 10 (58.82) | 14 (77.78) | 11 (64.71) | 13 (72.22) | |||
| TNM staging | 0.024 | 0.044 | |||||
| I+II stage (n=12) | 9 (52.94) | 3 (16.67) | 3 (17.65) | 9 (50.00) | |||
| III+IV Stage (n=23) |
8 (47.06) | 15 (83.33) | 14 (82.35) | 9 (50.00) | |||
| Lymphatic metastasis | 0.044 | 0.003 | |||||
| Transferred (n=12) | 3 (17.65) | 9 (50.00) | 10 (58.82) | 2 (11.11) | |||
| Non- Transferred (n=23) |
14 (82.35) | 9 (50.00) | 7 (41.18) | 16 (88.89) | |||
Table 4.
Analysis of Prognostic Factors in Patients with Gastric Cancer
| Factor | Single Factor Cox Regression | Multivariate Cox Regression | ||||
|---|---|---|---|---|---|---|
| P value | HR value | 95CI% | P value | HR value | 95CI% | |
| Gender | 0.780 | 0.886 | 0.380–2.066 | |||
| Age | 0.212 | 1.674 | 0.746–3.755 | |||
| Tumor size | 0.043 | 0.432 | 0.191–0.974 | 0.167 | 0.457 | 0.151–1.387 |
| Degree of differentiation | 0.040 | 2.844 | 1.051–7.697 | 0.053 | 2.799 | 0.988–7.933 |
| TNM staging | 0.030 | 3.001 | 1.116–8.070 | 0.035 | 3.336 | 1.088–10.228 |
| Lymphatic metastasis | 0.023 | 0.392 | 0.174–0.881 | 0.438 | 0.705 | 0.291–1.706 |
| miR26a5p | 0.033 | 2.431 | 1.074–5.502 | 0.768 | 0.850 | 0.288–2.508 |
| Wnt5a | 0.047 | 2.250 | 1.011–5.006 | 0.256 | 1.683 | 0.686–4.130 |
The Relationship Between miR-26a-5p, Wnt5a and the Survival and Prognosis of Patients with GC
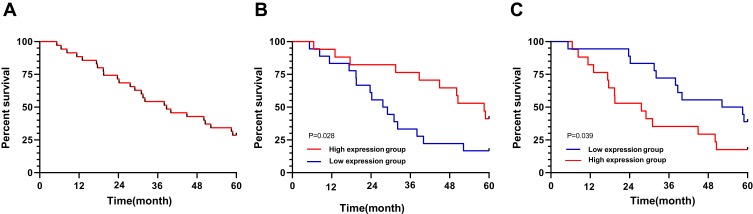
The patients were followed up for a duration of 5 years, with a five-year survival rate of 28.57% (10 cases). Judged by the median values of miR-26a-5p and Wnt5a expressions, the patients were divided into high and low expression groups. The 5-year survival rate of the patients with high expression of miR-26a-5p and low expression of Wnt5a was significantly higher than that of the corresponding group (PmiR-26a-5p=0.028, PWnt5a=0.039), as shown in Figure 3.
Figure 3.
The relationship between miR-26a-5p, Wnt5a expressions and the survival of patients with gastric cancer. (A) The five-year survival rate of gastric cancer patients; (B) Comparison of five-year survival of patients with high and low expression of miR-26a-5p; (C) Comparison of five-year survival of patients with high and low expression of Wnt5a.
The Expression of miR-26a-5p in GC Cells and Its Effect on Cell Biological Function
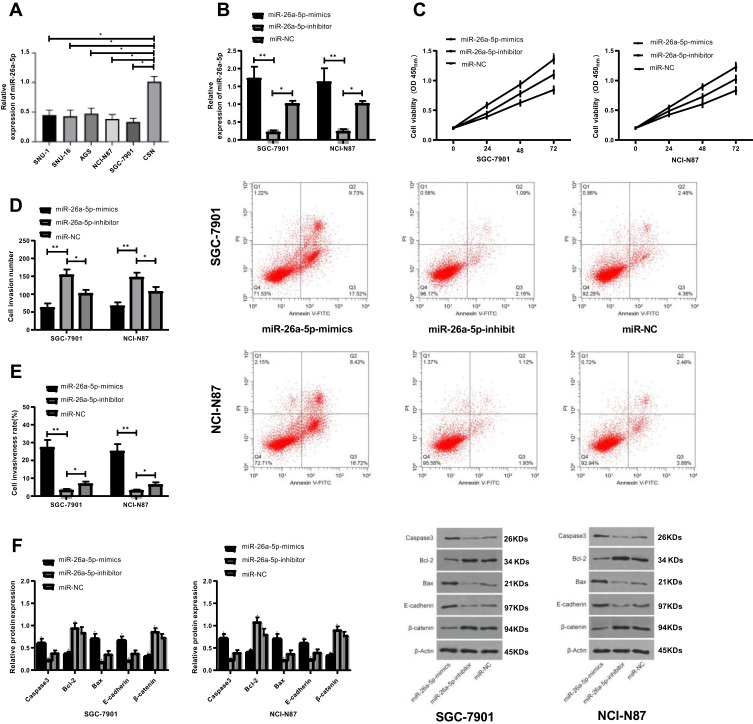
Detection of the expression of miR-26a-5p in GC cell lines showed that the expression of miR-26a-5p in all cancer cells was decreased than that in normal cells (P<0.05). The expression of microRNA-NC in SGC-7901 and NCI-N87 cells was detected after transfection. It was found that the expression of miR-7901 after transfection of miR-26a-5p-mics was higher than that of miR-26a-5p in NCI-N87 cells. Compared with the miR-NC group, the proliferation and invasion ability of SGC-7901 and NCI-N87 cells decreased and the apoptotic rate increased significantly after miR-26a-5p-mimics transfection. WB assay demonstrated that the relative expression of Bcl-2 and β-catenin protein was significantly decreased, while the relative expression of Caspase 3, E-cadherin and Bax protein was significantly increased (P<0.05). However, the results after transfection with miR-26a-5p-inhibitor were opposite to those after transfection with miR-26a-5p-mimics, with significant differences in cell biological function and protein expression (P<0.05), as shown in Figure 4.
Figure 4.
The expression of miR-26a-5p in gastric cancer cells and its effect on cell biological function. (A) Expression of miR-26a-5p in different groups; (B) Expression of miR-26a-5p in transfected SGC-7901 and NCI-N87 cells; (C) Comparison of proliferation between transfected SGC-7901 and NCI-N87 cells; (D) Comparison of invasive ability between transfected SGC-7901 and NCI-N87 cells; (E) Comparison of apoptosis rate between transfected SGC-7901 and NCI-N87; (F) Comparison of Caspase 3, Bcl-2, Bax, E-cadherin and β-catenin proteins in transfected SGC-7901 and NCI-N87 cells. *Represented the comparison with the miR-NC group, P<0.05. **Represented the comparison with the miR-NC group, P<0.01.
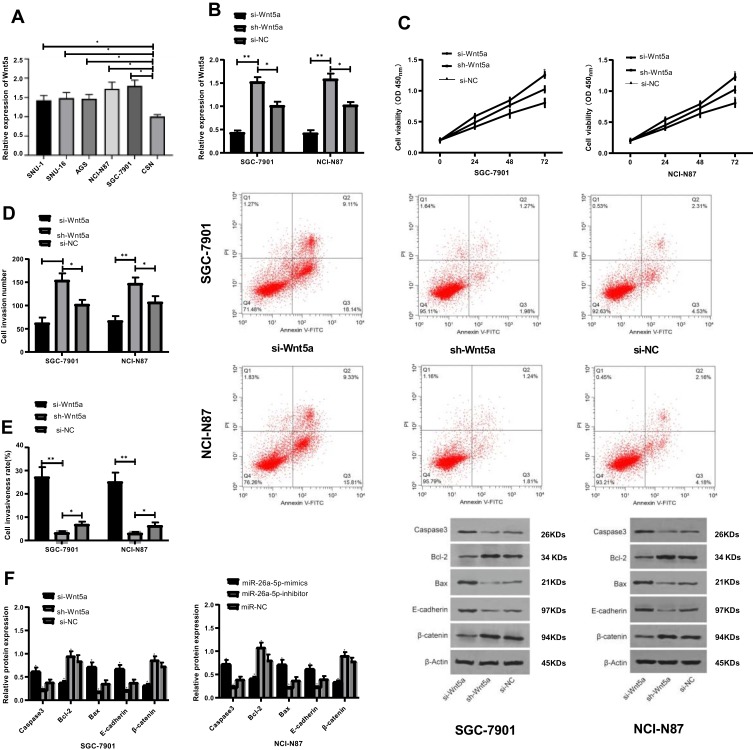
The Expression of Wnt5a in GC Cells and Its Effect on Cell Biological Function
The expression of Wnt5a in all cancer cells was higher than that in normal cells (P<0.05). The expression of SGC-7901 and NCI-N87 cells transfected with si-Wnt5a was tested and found significantly increased compared with that of the si-NC group (P<0.05), while the proliferation and invasion ability decreased and the apoptosis rate increased significantly. WB assay showed that the relative expression of Bcl-2 and β-catenin protein decreased significantly after transfection of si-Wnt5a, while the relative expression of Caspase 3, E-cadherin and Bax protein increased significantly (P<0.05). While the results of sh-Wnt5a transfection were opposite to those of si-Wnt5a transfection, which is significantly different (P<0.05), as shown in Figure 5.
Figure 5.
The expression of Wnt5a in gastric cancer cells and its effect on cell biological function. (A) Expression of Wnt5a in all groups of cells; (B) Expression of Wnt5a in transfected SGC-7901 and NCI-N87 cells; (C) Comparison of proliferation between transfected SGC-7901 and NCI-N87 cells; (D) Comparison of invasive ability between transfected SGC-7901 and NCI-N87 cells; (E) Comparison of apoptosis rate between transfected SGC-7901 and NCI-N87; (F) Comparison of Caspase 3, Bcl-2, Bax, E-cadherin and β-catenin proteins in transfected SGC-7901 and NCI-N87 cells. *Represented the comparison with the miR-NC group, P<0.05. **Represented the comparison with the miR-NC group, P<0.01.
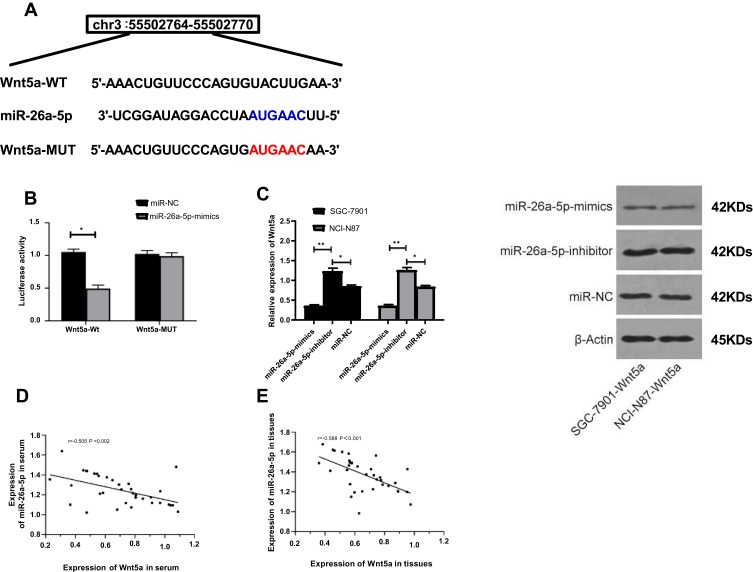
MiR-26a-5p Targets Wnt5a in GC Cells
The analysis of Targetscan7.2, starBase 2.0, miRBD and miRTarBase online prediction websites found possible potential binding targets between miR-26a-5p and Wnt5a. In order to further confirm the relationship between the two, the dual luciferase reporter assay was conducted, and the results showed that the over-expression of miR-26a-5p significantly reduced the luciferase activity of pmirGLO-Wnt5a-3ʹUT Wt (P<0.05), but had no effect on that of pmirGLO-Wnt5a-3ʹUT Mut (P>0.05). WB assay demonstrated that the expression of Wnt5a protein in cells transfected with miR-26a-5p-mimics and miR-26a-5p-inhibitor was significantly different from that of microRNA-NC (P<0.05). The correlation analysis revealed a negative correlation between miR-26a-5p and Wnt5a in serum and tissues of patients, as shown in Figure 6.
Figure 6.
Target gene identification of miR-26a-5p. (A) miR-26a-5p binds to Wnt5a and mutates; (B) Dual-Luciferase reporter assay confirms targeted binding of miR-26a-5p to Wnt5a; (C) Expression of Wnt5a protein in SGC-7901 and NCI-N87 cells after transfection of miR-26a-5pmimics and miR-26a-5p-inhibit; (D) Analysis of the correlation between miR-26a-5p and Wnt5a in patients’ serum; (E) Analysis of the correlation between miR-26a-5p and Wnt5a in patients’ tissues. *Represented P<0.05, and **Represented P<0.001.
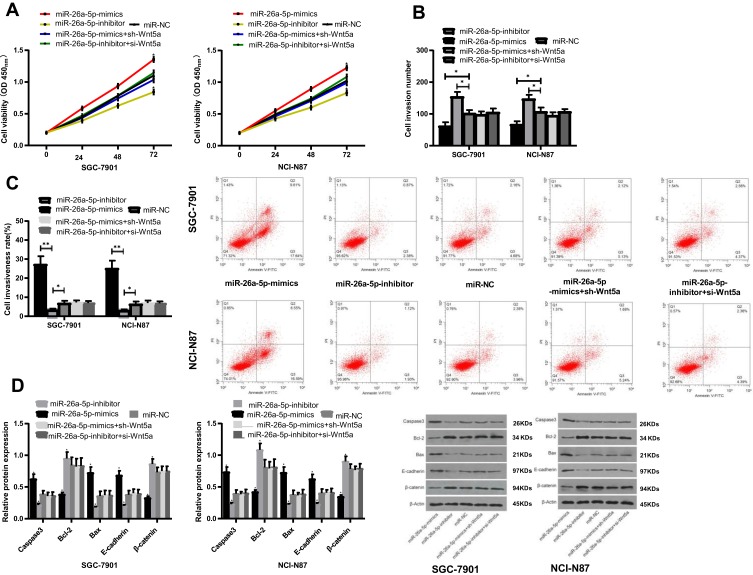
Rescue Experiments
Mi-NC, Mi-26a-5p-mimics+sh-Wnt5a, Mi-26a-5p-inhibitor+si-Wnt5a, Mi-26a-5p-mics, and Mi-26a-5p-inhibitor were transfected into SGC-7901 and NCI-N87 cells, respectively. The results showed that the cell proliferation and invasion, as well as the apoptosis rate of the transfected miR-26a-5p-mimics+sh-Wnt5a and the transfected miR-26a-5p-inhibitor+si-Wnt5a was not significantly different with that of the mir-NC group. However, compared with miR-26a-5p-mimics transfected cells, the cell proliferation and invasion ability were increased, and the apoptosis rate was significantly reduced. While the cell proliferation and invasion were reduced, and apoptosis rate was significantly increased when compared with miR-26a-5p-inhibitor cells. WB assay showed that the expression of Wnt5a, Caspase 3, Bcl-2, Bax, E-cadherin, and beta-nin protein in cells after transfection with microRNA-NC, miR-26a-5p-mimics + sh-Wnt5a, miR-26a-5p-inhibitor + si-Wnt5a were not significantly different. Compared with miR-26a-5p-mimics, the relative expression of Wnt5a, Bcl-2, β-catenin protein was significantly increased, while the relative expressions of Caspase3, E-cadherin and Bax proteins were significantly decreased. The results of biological function and protein expression of miR-26a-5p-mimics were opposite to those of the transfected miR-26a-5p-mimics, with significant differences (P<0.05), as shown in Figure 7.
Figure 7.
Rescue experiment. (A) Effects of transfection with miR-26a-5p-mimics+sh-Wnt5a and miR-26a-5p-inhibitor+si-Wnt5a on proliferation of SGC-7901 and NCI-N87 cells; (B) Effects of transfection with miR-26a-5p-mimics+sh-Wnt5a and miR-26a-5p-inhibitor+si-Wnt5a on the invasive function of SGC-7901 and NCI-N87 cells; (C) Effect of transfection with miR-26a-5p-mimics+sh-Wnt5a and miR-26a-5p-inhibitor+si-Wnt5a on the apoptosis rate of SGC-7901 and NCI-N87 cells; (D) Effects of transfection with miR-26a-5p-mimics+sh-Wnt5a and miR-26a-5p-inhibitor+si-Wnt5a on apoptosis proteins of SGC-7901 and NCI-N87 cells. *Represented the comparison with the miR-NC group, P<0.05. **Represented the comparison with the miR-NC group, P<0.01.
Discussion
As a common malignant tumor of digestive system worldwide, gastric cancer (GC) is one of the leading factors of cancer-related death.22 However, little progress has been made in the treatment and prognosis of GC patients to date,23 which is mainly resulting from the fact that the pathogenesis of GC is still unclear and there is a lack of biomarkers for early diagnosis of GC. Therefore, it is of great importance to further elucidate the relevant mechanism and explore potential biomarkers for the diagnosis of GC to treat patients and improve prognosis.
As mentioned earlier, miR is a class of conservative short non-coding RNAs, with a length of about 21-25nt which are implicated in a variety of biological pathways in the body and can complement each other by targeting the 3ʹUTR, thus regulating target gene transcription and reducing the expression of the gene.23–25 Guo et al26 have found that overexpression of miR-634 can inhibit the expression of JAG1 protein and inhibit the proliferation, migration and invasion of GC cells. MiR-26a-5p, as the earliest discovered class of microRNAs, is located on human 3p22.2 chromosome; however, its correlation with GC remains elusive. As we all know, metastasis of GC poses a major clinical challenge, presenting a poor prognosis and limited treatment options for patients. Therefore, reducing the occurrence of metastasis is an effective means to better patients’ prognosis.
The TCGA database analysis revealed that miR-26a-5p was underexpressed in GC tissues, and Wnt5a was a potential target of miR-26a-5p through multiple online miR target gene prediction sites. Then, we re-validated their relation by finding that Wnt5a was highly expressed in GC tissues through TCGA database, indicating that miR-26a-5p and Wnt5a were expected to be potential therapeutic targets for GC. Furthermore, the expression of miR-26a-5p and Wnt5a in the serum and tissues of clinical patients was found to be consistent with those in the database. Moreover, according to ROC curve analysis, miR-26a-5p and Wnt5a were of high value in the diagnosis of GC. Additionally, patients with low expression of miR-26a-5p and high expression of Wnt5a were found to present significantly increased incidence of stage III+IV, lymphatic metastasis and tumor ≥3cm, with some certain diagnostic value. As a non-classical ligand of Wnt pathway,27 Wnt5a was identified as a non-classical ligand in the Wnt family and was highly expressed in GC and other cancers,28,29 which was consistent with our study. Last but not the least, judged by the median value of miR-26a-5p and Wnt5a, patients were divided into high and low expression groups, through which it was found that patients with high expression of miR-26a-5p and low expression of Wnt5a had significantly higher survival rates in 3 and 5 years than their corresponding groups, suggesting that these two indicators can both be used as potential prognostic indicators for GC. This study is therefore set out to determine the relevant mechanism of miR-26a-5p and Wnt5a in patients with GC through relevant basic experiments.
The expression of miR-26a-5p and Wnt5a in different cells was detected and it was found that the two genes expressed most significantly in NCI-N87 and SGC-7901 cells. Therefore, the overexpression and inhibition sequences were transfected into these two cell lines, respectively. The proliferation and invasion of the cells transfected with miR-26a-5p-mics and si-Wnt5a were significantly inhibited, while the expression of Bcl-2 and β-catenin decreased, Caspase 3, E-cadherin and Bax increased. The proliferation and invasion of cells transfected with miR-26a-5p-inhibit + sh-Wnt5a increased significantly, while the apoptosis rate decreased, the expression of Bcl-2 and β-catenin increased, and the expression of Caspase 3, E-cadherin and Bax protein reduced. The dual luciferase reporter assay showed that the binding of miR-26a-5p to Wnt5a could be targeted. WB assay indicated that the expression of Wnt5a protein decreased after the transfection of miR-26a-5p-mimics, while increased after the transfection of miR-26a-5p-inhibitor. Analysis of the expression of miR-26a-5p and Wnt5a in serum and tissue of clinical patients showed a negative correlation. It was preliminarily confirmed that over-expression of miR-26a-5p could inhibit the expression of Wnt5a protein and regulate the biological function of cells. At the end of the study, rescue experiments demonstrated that the proliferation, invasion and apoptosis of the cells transfected with miR-26a-5p-mimics+sh-Wnt5a and miR-26a-5p-inhibitor+si-Wnt5a were not significantly different from those of the microRNA-NC group. The biological function of the cells transfected with both miR-26a-5p-mimics and miR-26a-5p-inhibit was significantly different from that of transfected by either of them alone. This study has clarified the role of miR-26a-5p and Wnt5a in GC, but there are still some limitations.
First of all, nude mice experiment was not carried out in this study, so it is not clear whether miR-26a-5p affects the volume change of tumors. Secondly, due to the small sample size of this clinical study, which may also be one of the factors leading to little difference of miR-26a-5p and Wnt5a in Cox regression multivariate analysis, the authority of the experimental results still needs further large-scale clinical research to verify. Nevertheless, we will carry out more basic experiments and collect more clinical samples to further verify and supplement our research results.
Conclusion
To sum up, miR-26a-5p was found to be highly expressed in serum and tissues of patients with GC, and by upregulation of miR-26a-5p, it can inhibit the increase of Wnt5a, tumor proliferation and invasion, and promote cell apoptosis, which is expected to be a potential target for clinical treatment.
Disclosure
Yu Li and Peng Wang contributed equally to this study as co-first authors. The authors report no conflicts of interest in this work.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.v68.6 [DOI] [PubMed] [Google Scholar]
- 3.Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–v49. doi: 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 4.Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oki E, Tokunaga S, Emi Y, et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer. 2016;19(3):968–976. doi: 10.1007/s10120-015-0530-z [DOI] [PubMed] [Google Scholar]
- 6.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37. doi: 10.1038/s41580-018-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16(7):421–433. doi: 10.1038/nrg3965 [DOI] [PubMed] [Google Scholar]
- 8.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–333. doi: 10.1038/nrc3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng D, Wang L, Chen Y, et al. MicroRNA-124-3p regulates cell proliferation, invasion, apoptosis, and bioenergetics by targeting PIM1 in astrocytoma. Cancer Sci. 2016;107(7):899–907. doi: 10.1111/cas.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv C, Hao Y, Tu G. MicroRNA-21 promotes proliferation, invasion and suppresses apoptosis in human osteosarcoma line MG63 through PTEN/Akt pathway. Tumour Biol. 2016;37(7):9333–9342. doi: 10.1007/s13277-016-4807-6 [DOI] [PubMed] [Google Scholar]
- 11.Hannafon BN, Trigoso YD, Calloway CL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18(1):90. doi: 10.1186/s13058-016-0753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callegari E, Gramantieri L, Domenicali M, D’Abundo L, Sabbioni S, Negrini M. MicroRNAs in liver cancer: a model for investigating pathogenesis and novel therapeutic approaches. Cell Death Differ. 2015;22(1):46–57. doi: 10.1038/cdd.2014.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasinski AL, Kelnar K, Stahlhut C, et al. A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene. 2015;34(27):3547–3555. doi: 10.1038/onc.2014.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanbari R, Mosakhani N, Asadi J, et al. Downregulation of plasma MiR-142-3p and MiR-26a-5p in patients with colorectal carcinoma. Iran J Cancer Prev. 2015;8(3):e2329. doi: 10.17795/ijcp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu X, Zhang J, Shi W, et al. Circulating MicroRNA-26a in plasma and its potential diagnostic value in gastric cancer. PLoS One. 2016;11(3):e0151345. doi: 10.1371/journal.pone.0151345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asem MS, Buechler S, Wates RB, Miller DL, Stack MS. Wnt5a signaling in cancer. Cancers (Basel). 2016;8(9):79. doi: 10.3390/cancers8090079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takiguchi G, Nishita M, Kurita K, Kakeji Y, Minami Y. Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci. 2016;107(3):290–297. doi: 10.1111/cas.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Wang Q, Wang YA, et al. Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell. 2016;167(5):1281–1295 e1218. doi: 10.1016/j.cell.2016.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang F, Xu S, Wang G, Kuang D. Expression of Wnt5a and Wnt11 in lung cancer and their significance. Chin J Clin Exp Pathol. 2017;33(10):1082–1086. [Google Scholar]
- 20.In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann Surg Oncol. 2017;24(12):3683–3691. doi: 10.1245/s10434-017-6078-x [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods (San Diego, Calif). 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 22.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to helicobacter pylori. Int J Cancer. 2015;136(2):487–490. doi: 10.1002/ijc.28999 [DOI] [PubMed] [Google Scholar]
- 23.Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47(D1):D155–D162. doi: 10.1093/nar/gky1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seitz H. On the number of functional microRNA targets. Mol Biol Evol. 2019;36(7):1596–1597. doi: 10.1093/molbev/msz054 [DOI] [PubMed] [Google Scholar]
- 25.Szasz AM, Lanczky A, Nagy A, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7(31):49322–49333. doi: 10.18632/oncotarget.10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J, Zhang CD, An JX, et al. Expression of miR-634 in gastric carcinoma and its effects on proliferation, migration, and invasion of gastric cancer cells. Cancer Med. 2018;7(3):776–787. doi: 10.1002/cam4.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi YN, Zhu N, Liu C, et al. Wnt5a and its signaling pathway in angiogenesis. Clin Chim Acta. 2017;471:263–269. doi: 10.1016/j.cca.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Yu Z, Tan Q, et al. MiR-876-5p regulates gastric cancer cell proliferation, apoptosis and migration through targeting WNT5A and MITF. Biosci Rep. 2019;39:6. doi: 10.1042/BSR20190066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam S, Chung JW, Yang JY. WNT5A correlates with clinicopathological characteristics in gastric cancer: a meta-analysis. Cell Physiol Biochem. 2017;41(1):33–40. doi: 10.1159/000455934 [DOI] [PubMed] [Google Scholar]