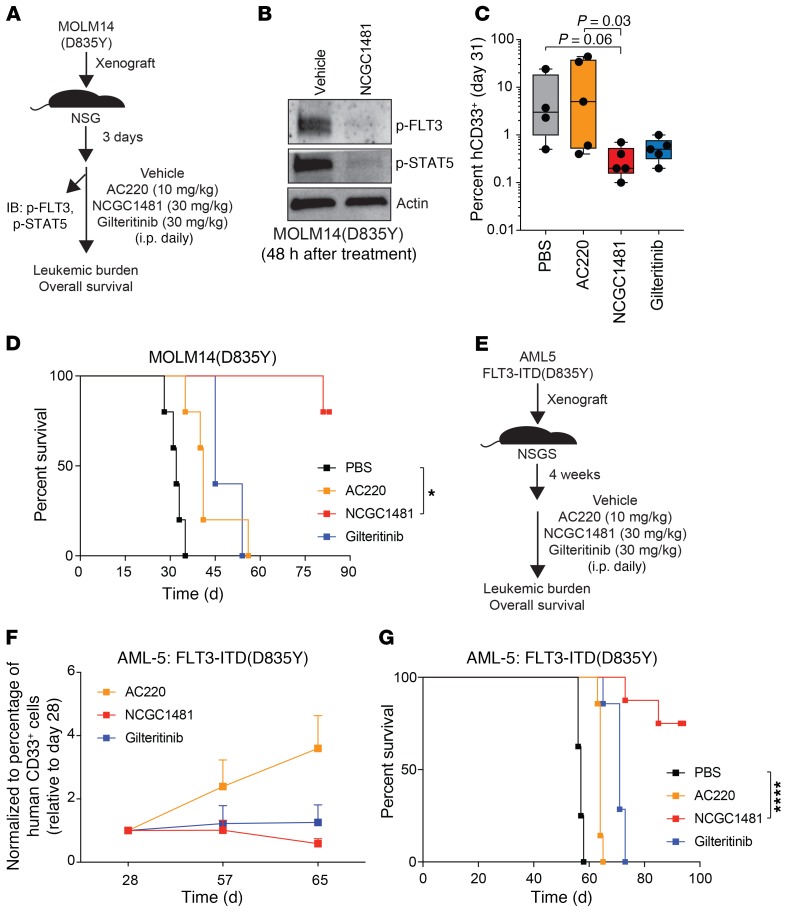
Figure 3. NCGC1481 targets clinically relevant FLT3-mutant AML cells in vivo.
(A) Experimental design of the xenograft studies. (B) After treatment with NCGC1481, MOLM14-FLT3-ITD(D835Y) cells (human CD33+ [hCD33+]) were isolated from the BM for immunoblot analysis. (C) BM aspirates were analyzed for leukemic burden on day 31 after transplantation (n = 5 mice per condition). Values are expressed as the mean ± SEM for individual mice. P = 0.06 for PBS versus NCGC1481; P = 0.03 for AC220 versus NCGC1481; Dunn’s multiple comparisons test. (D) Leukemia-free survival of NSG mice xenografted with MOLM14-FLT3-ITD(D835Y) cells and treated with the indicated FLT3i or vehicle (n = 5 mice per group). *P < 0.05, by log-rank (Mantel-Cox) test. (E) Experimental design of patient-derived xenograft studies. (F) BM was analyzed for leukemic burden around day 65 after transplantation (n = 5–7 mice per condition). Values are expressed relative to baseline values on day 28 for individual mice. (G) Leukemia-free survival of NSGS mice xenografted with AML-5 cells and treated with the indicated FLT3i or vehicle (n = 7–8 mice per group). ****P < 0.0001, by log-rank (Mantel-Cox) test.