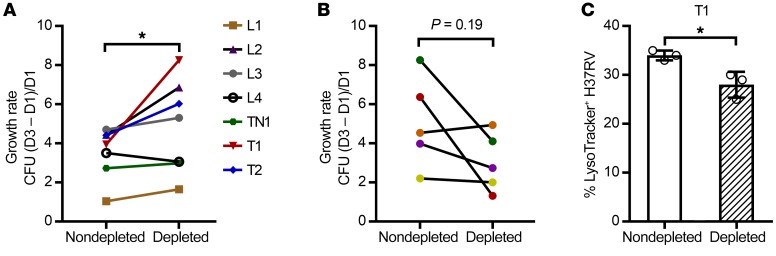
Figure 8. Protective in vitro efficacy seen with anti-AM Abs from asymptomatic individuals with BCG or Mtb exposure/infection but not with anti-AM Abs from TB patients.
Effects of nondepleted versus anti-AM Ab–depleted sera on intracellular Mtb growth (H37Rv; MOI 1) in human macrophages (THP-1), indicating that anti-AM Abs from asymptomatic subjects reduce while those from TB patients enhance intracellular Mtb growth. Experiment repeated with selected sera showed the same results. Sera depleted with control (BSA-coupled) beads had no effects on Mtb intracellular growth compared with nondepleted sera (Supplemental Figure 4). (A) Differences in intracellular growth rates with sera from TST+IGRA+ (L, n = 4) and TST+IGRA– (T, n = 2) or TST– (TN, n = 1) subjects with the highest anti-AM IgG titers. Wilcoxon matched-pairs signed-rank test. (B) Differences in intracellular growth rates with sera from TB patients with high anti-AM IgG titers (n = 5; all pulmonary TB). Note that our power was limited because only 5 high-titer sera with sufficient volume were available before the start of antituberculous therapy. (C) High–anti-AM IgG serum (T1) enhanced phagolysosomal fusion in Mtb-infected THP-1 cells (MOI 5) significantly more than anti-AM Ab–depleted serum. Columns and error bars represent mean and SD of triplicates (open circles). Unpaired t test. *P < 0.05.