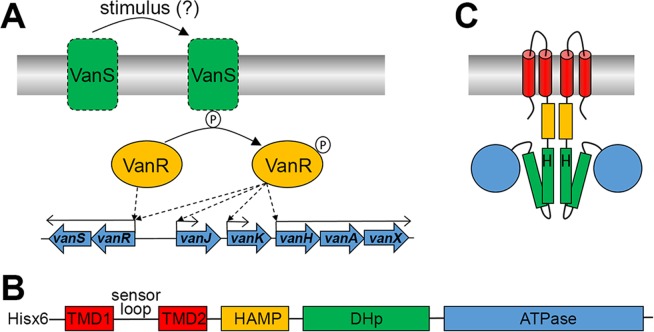
Figure 1.
(A) The regulatory role of VanSR on vanHAX transcription in Actinomycetes. VanS is stimulated, in the presence of vancomycin, to undergo autophosphorylation and subsequent phosphotransfer to VanR. Compared to VanR, Phospho-VanR has an increased binding affinity for the promoter regions of vanR as well as vanH, vanJ and vanK. In the absence of vancomycin, VanR remains dephosphorylated, and binds only to the promoter region of vanR to maintain low-level expression of VanSR. (B) Predicted architecture of VanS from S. coelicolor (VanSSC) based on homology modelling. The extracellular sensor loop domain is flanked by two transmembrane domains (TMD 1–2), followed by an intracellular HAMP domain, a dimerisation and histidine phosphorylation (DHp) domain which is the site of autophosphorylation via a conserved histidine, and an ATPase domain. Location of the amino terminal hexahistidine tag on the protein described in this study is also indicated. (C) Predicted topology of the domains in VanSSC.