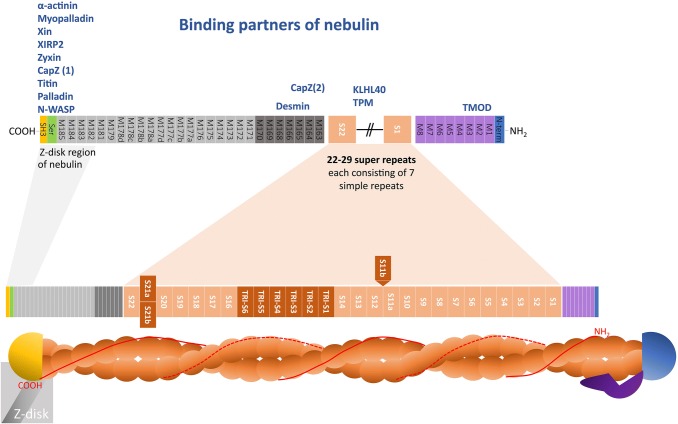
Fig. 2.
Nebulin protein structure and binding partners. The majority of nebulin’s protein sequence consists of repetitive modules (M) called simple repeats which correspond to actin binding sites. The central region is further organised into super-repeats made up of seven simple repeats each (orange). The composition of the central super-repeat region is strongly affected by alternative splicing, varying the number of super-repeats in the produced protein from 22 to 29. Three main areas have been found to be alternatively spliced (shown in dark orange): (1) exons 63–66 (encoding S11b; transcripts were found to either contain or lack this super-repeat); (2) exons 82–105 [the triplicate region of nebulin encodes 6 super-repeats (TRI-S1 to TRI-S6); the exact splicing pattern has not been established]; (3) exons 143/144 (encoding S21a or S21b; both exons have not been detected in the same transcript). Within the central super-repeat region nebulin is thought to interact with tropomyosin and KLHL40 (listed above in blue). The N- and C-terminus of nebulin are not organised into super-repeats. The C-terminus is made up of two distinct versions of repeats called linker repeats (M163–M170; dark grey) and simple repeats (M171–M183; light grey) (Labeit and Kolmerer 1995). Nebulin’s C-terminus localises within the Z-disk of the sarcomere, likely from M182. A serine-rich region and a highly conserved Src homology-3 (SH3) domain are located at the C-terminus of nebulin and mediate interactions with a large number of proteins (listed above in blue). The N-terminus of nebulin is close to the pointed end of the thin filament. The first 77 N-terminal residues contain a unique, glutamic acid rich sequence (blue) followed by repeat M1–M8 (purple) which are distinct from the remaining repeats and important to mediate tropomodulin interactions at the thin filament pointed end. (Color figure online)