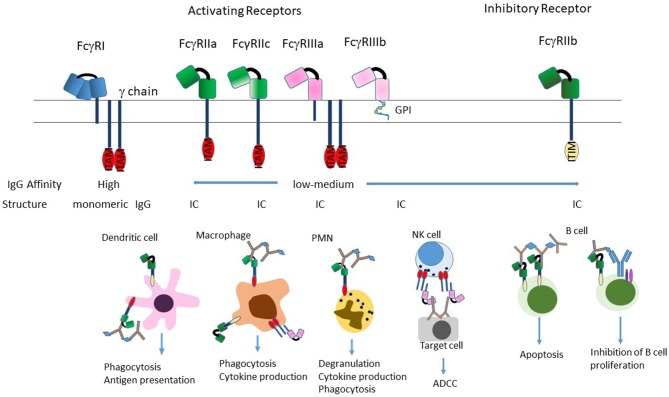
Figure 2.
Structure of FcγRs. The FcγR's differ in their affinity for IgG; FcγRI is a high-affinity receptor and is the only one that can effectively bind monomeric IgG; the two low-affinity receptors FcγRII and FcγRIII preferentially bind IgG in the form of immune complexes. FcγRI and FcγRIIIa exist as transmembrane proteins each non-covalently linked to a common FcRγ subunit. The γ subunit exists as a homodimer containing an immunoreceptor tyrosine-based activation motif (ITAM) within its intracellular domain. FcγRII exists on the cell surface as a single chain with the ligand-binding region in the extracellular domain and either an ITAM (FcγRIIa), or an immunoreceptor tyrosine-based inhibition motif (ITIM; FcγRIIb) in the intracytoplasmic domain necessary for signal transduction reviewed in (33). FcγRIIIb is the only receptor anchored to the membrane via a glycosylphosphatidylinisotol (GPI) link (34). Stimulation of the FcγRs by ICs induces a variety of effector functions that varies by cell type. Most cells express a combination of activating and inhibitory receptors, which allows fine-tuning of the response to ICs. The exception to this is NK cells, which only express activating receptors, and B cells that only express the inhibitory receptor.