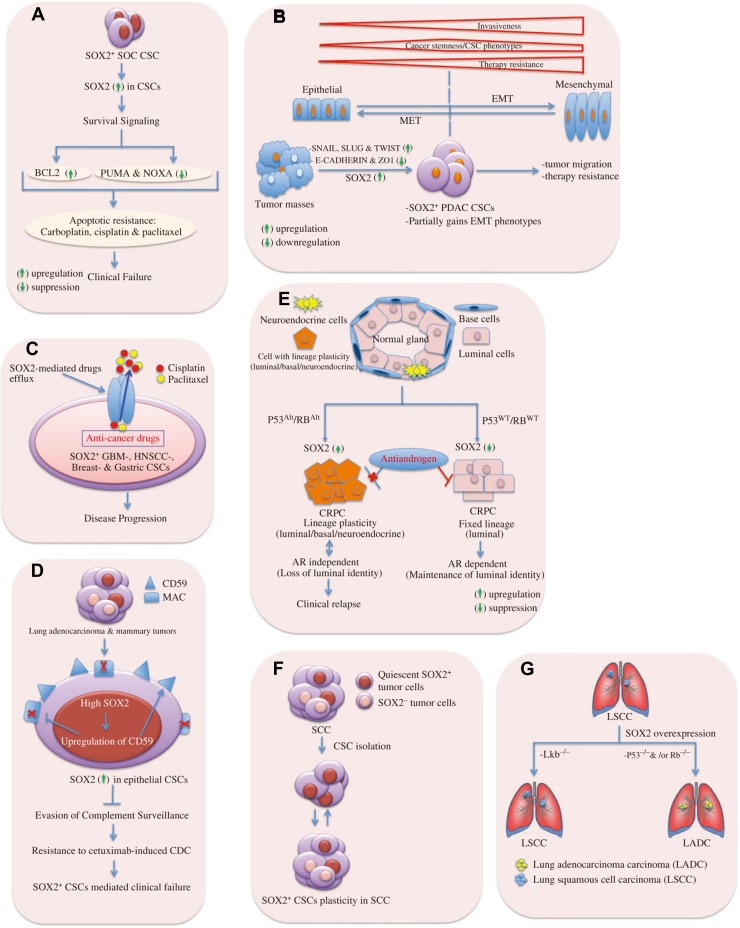
Figure 2.
Mechanistic links to SOX2-dependent CSC-mediated clinical relapse. (A) SOX2 mediates survival signal to CSCs. In SOC CSCs, elevated SOX2 expression is associated with upregulation of anti-apoptotic factor BCL2 and suppression of pro-apoptotic proteins PUMA and NOXA. This provides survival signal to persist under anti-cancer drugs as carbolatin, cisplatin, or paclitaxel, and thus, enhancing apoptotic resistance. (B) CSCs gain partial EMT phenotypes facilitating tumor malignancy due to SOX2 expression. It illustrates the extent of invasiveness, tumor-initiating ability, and a change in degree of drug resistance across the spectrum of EMT-program activation. Tumor invasiveness and drug resistance increase upon gaining complete EMT phenotypes. The cancer stemness or tumor-initiating ability of carcinoma cells is influenced by the level of EMT-program activation and it peaks at an intermediate level of EMT. Indeed, extensive EMT activation is usually detrimental to tumor-initiating ability. The drug resistance of carcinoma cells also seems to be maximal at an intermediate level of EMT-program activation, but plateaus (rather than declines) with further activation of this program. In pancreatic adenocarcinoma (PADC), SOX2 expression imparts partial EMT-like phenotypes to PADC CSCs via upregulation of the EMT master regulators SNAIL, SLUG, and TWIST, and it is shown that SOX2 cannot induce fully EMT program in PADC. Thus, SOX2 contributes to EMT-mediated tumor malignancy. MET, mesenchymal-to-epithelial transition. (C) SOX2 mediates drug efflux in CSCs. SOX2 in CSCs enhances the expression of ABC drug transporters that can effectively efflux anti-cancers drugs (e.g. cisplatin, and paclitaxel) from glioblastoma (GBM), breast cancer, gastric cancer, and HNSCC CSCs through hydrolysis of ATP. Thus, CSCs acquire resistance to therapy and cause clinical failure. CSCs are likely to share many properties of normal stem cells providing an opportunity for a long lifespan, e.g. they remain relative quiescence, show resistance to drugs, and efflux toxins through expression of ABC transporters. This points to the tumors having built-in population of drug-resistant pluripotent cells that can survive chemotherapy and repopulate the tumor. (D) SOX2 mediates evasion of complement surveillance by CSCs. High SOX2 expression in epithelial CSCs causes upregulation of CD59, which in turn leads to inhibition of membrane attack complex (MAC). Thus, CSCs avoid complement attacks and show enhanced resistance to CDC. (E) SOX2 promotes lineage plasticity in p53−/− and Rb−/− prostate cancers. SOX2 is responsible for anti-androgen resistance in castration-resistant prostate cancers of adenocarcinoma histology (CRPC-adeno) due to TP53 and RB1 alterations (TP53Alt, RB1Alt) compared to those with WT TP53 and RB1 (TP53WT, RB1WT). High SOX2 expression leads to anti-androgen drug resistance (e.g. enzalutamide) in CRPC-adeno upon loss of tumor suppressor genes p53 and Rb. Luminal identity is characterized by the presence of androgen receptor (AR). The tumors can develop resistance to the anti-androgen drug by a phenotypic shift from androgen receptor (AR)-dependent luminal epithelial cells to AR-independent basal-like cells with mixed phenotypes (luminal/basal/neuroendocrine cells). (F) SOX2 provides lineage plasticity to CSCs. In skin SCC, SOX2+ tumor-propagating cells give rise to both SOX2-expressing and SOX2-negative tumor cell progenies, thus, imparting plasticity to CSCs. (G) SOX2 involves in lineage-specific survival mechanism. SOX2 overexpression gives rise to LSCC upon loss of the tumor suppressor Lkb or LADC upon loss of p53 and/or Rb.