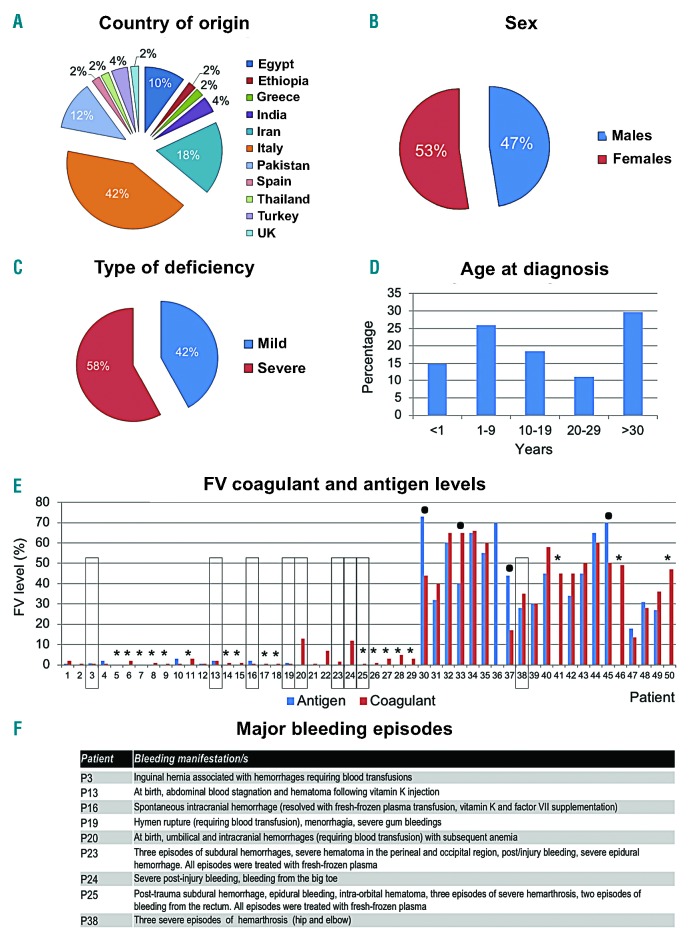
Figure 1.
Demographic and clinical characteristics of the case series. Local Ethics Committees approved this study. All individuals (or their parents, if minor) signed an informed consent according to the Declaration of Helsinki. Data on the enrolled 50 coagulation factor V (FV)-deficient patients are presented as aggregated. Pie-charts and histograms illustrate the country of origin (A), the female to male ratio (B), the classification of patients according to the severity of the deficiency (C), and the distribution of age at diagnosis (D). Data on sex and age are missing for one patient. FV coagulant (FV:C) and FV antigen (FV:Ag) levels are detailed in panel (E), where the number of each patient is reported on the X axis, and corresponds to the numbering (P1-P50) reported in Table 1. FV:C and FV:Ag were measured as described;3 normal ranges for both tests are 60-140%. All patients were measured for FV:C, except P36 (for whom only FV:Ag is reported). Patients lacking FV:Ag measurements are indicated by an asterisk. Patients showing a discordance >30% between FV:C and FV:Ag are marked by a dot. In panel (F), the list of all major bleeding episodes, affecting nine patients (evidenced with a rectangle in panel E), is reported.