Fig. 1.
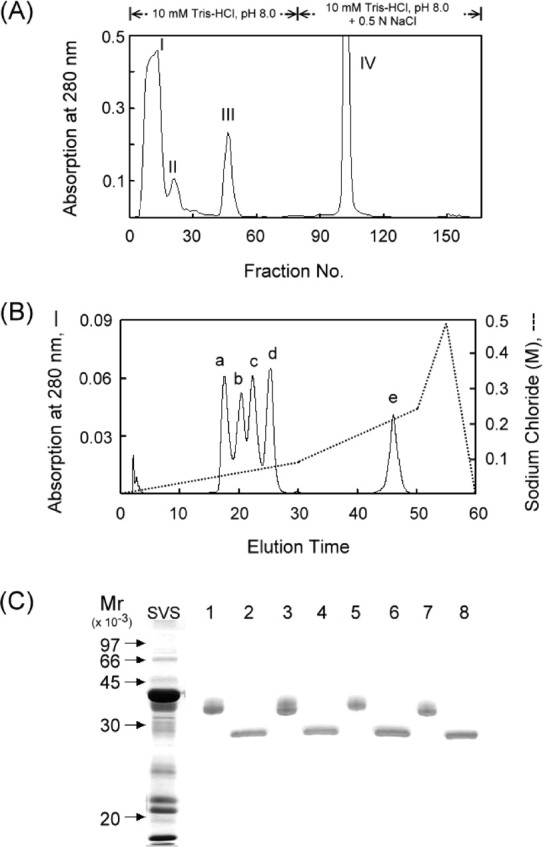
Purification of 36-kDa glycoproteins from mouse SVS proteins. A) Fractionation of soluble mouse SVS proteins by ion exchange chromatography on a DEAE-Sephacel column. B) Resolution of fraction III sample from A by ion-exchange HPLC on an SP column. C) Demonstration of the glycoprotein nature. Each of the a-to-d peaks from B were digested with N-glycosidase F. The parent proteins (lane 1, peak a; lane 3, peak b; lane 5, peak c; lane 7, peak d) and their deglycosylated forms (lanes 2, 4, 6, and 8) were identified by SDS-PAGE on a 12% polyacrylamide gel slab. The proteins in the gel were stained with Coomassie brilliant blue
