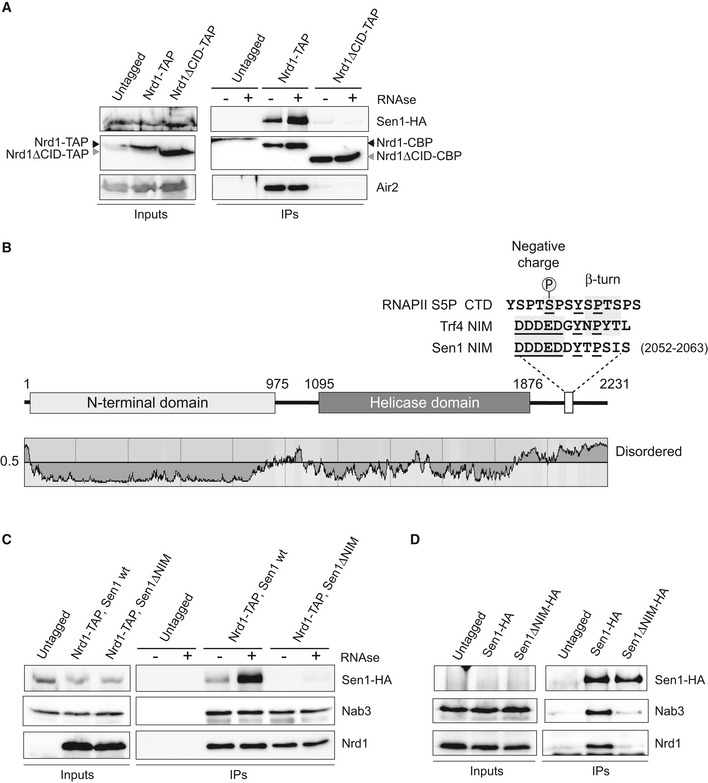
Figure 1. Identification of a Nrd1‐Interaction Motif (NIM) in Sen1 that is critical for the integrity of the NNS complex.
-
ADeletion of the CID domain dramatically reduces the interaction of Nrd1 with Sen1. Coimmunoprecipitation (CoIP) experiments using TAP‐tagged Nrd1 (either wt or ∆CID) as the bait. Representative gel of one out of two independent experiments.
-
BScheme of Sen1 protein. Globular domains are denoted by solid bars, whereas intrinsically disordered regions are shown by a line. The disorder prediction was obtained using IUPred (Dosztányi et al, 2005). The sequence of the RNAPII S5P‐CTD and Trf4 and Sen1 NIMs is shown on the top. Structural elements that are important for the interaction with Nrd1 CID are indicated. Conserved positions are underlined.
-
CDeletion of the NIM decreases substantially the association of Nrd1 with Sen1. CoIP experiments using Nrd1‐TAP as the bait in a SEN1 or sen1∆NIM background. Representative gel of one out of two independent experiments.
-
DCoIP experiments using HA‐tagged Sen1, either wt or ∆NIM, as the bait. Representative gel of one out of two independent experiments. Protein extracts were treated with RNaseA prior to immunoprecipitation. In these experiments, Sen1 could not be detected in the input extracts.
