Figure 5. The N‐terminal domain of Sen1 can recognize the S5‐phosphorylated form of RNAPII CTD and Sen1 C‐terminal domain.
-
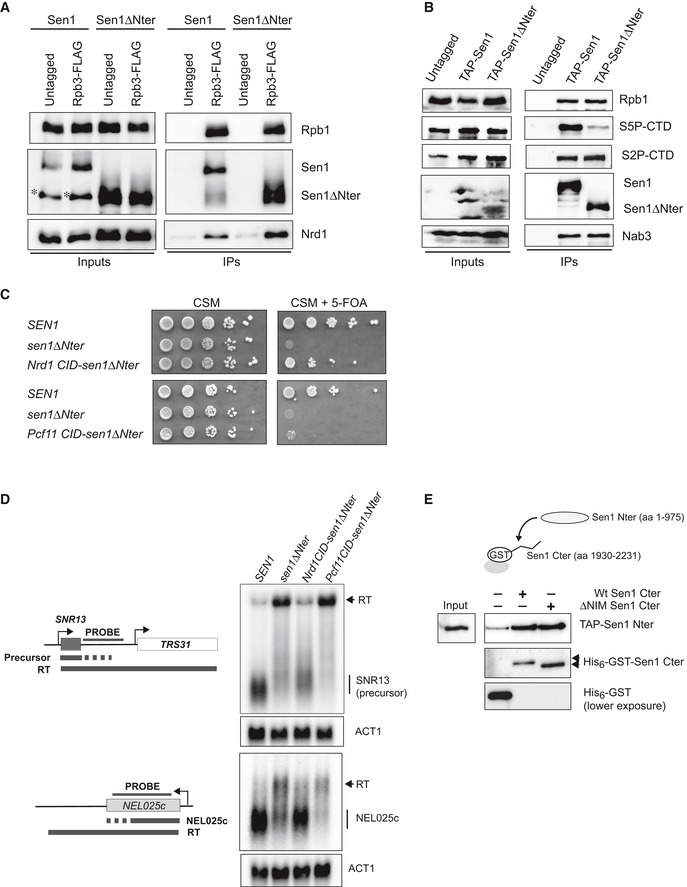
ADeletion of Sen1 N‐terminal domain does not prevent the interaction of Sen1 with RNAPII. CoIP experiments using Rbp3‐FLAG as the bait. Assays were performed in a Sen1‐AID strain harbouring a plasmid expressing either SEN1 or sen1∆Nter upon depletion of Sen1‐AID in the presence of IAA for 2 h. An asterisk denotes a major proteolytic Sen1 fragment detected in the extracts of roughly the size of sen1∆Nter. Nrd1 is detected as a positive control. Representative gel of one out of two independent experiments. Protein extracts were treated with RNaseA before immunoprecipitation.
-
BDeletion of the Sen1 N‐terminal domain reduces the interaction of Sen1 with the S5P‐CTD. CoIP experiments using TAP‐Sen1 as the bait. Sen1 proteins were expressed from pGAL in the presence of galactose. Nab3 is detected as a positive control. Representative gel of one out of three independent experiments. Protein extracts were treated with RNaseA before immunoprecipitation.
-
CReplacing the Nter of Sen1 by the CID of Nrd1 restores viability. Growth test performed in the same conditions as in Fig 3A but in the presence of a TRP1‐plasmid carrying the SEN1 versions indicated in the scheme on the left. The growth of the strain expressing the Nrd1 CID‐sen1∆Nter chimera in 5‐FOA implies that this gene can support viability.
-
DSubstituting the Nter of Sen1 by Nrd1 CID but not Pcf11 CID partially suppresses the termination defects detected in the sen1∆Nter mutant. Northern blot assays performed in a Sen1‐AID strain carrying an empty vector or a plasmid expressing the indicated versions of SEN1 upon depletion of the endogenous Sen1 protein as in Fig 3B. Experiments performed in a ∆rrp6 background. Representative gel of one out of two independent experiments. The U4 RNA is used as a loading control. Probes used for RNA detection are described in Appendix Table S6.
-
ESen1 Nter interacts with the C‐terminal domain (Cter) of Sen1 both in the presence and in the absence of the NIM in vitro. Pull‐down experiments using either a wt or a ∆NIM version of recombinant Sen1 Cter immobilized on glutathione sepharose beads and a TAP‐tagged version of Sen1 Nter expressed in yeast. Representative gel of one out of three independent experiments.
