Figure EV5. CENP‐C structure and binding to CAL1.
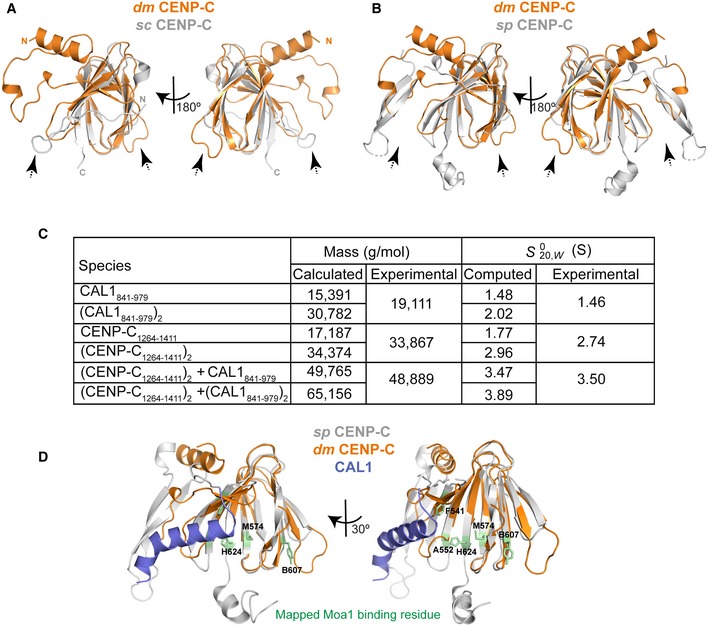
- Structural superposition of cupin domains of dm CENP‐C and sc CENP‐C (Mif2p) (Cohen et al, 2008). CENP‐C is shown orange and Mif2p in silver. Dotted arrow highlights conformational changes in the loop regions.
- Structural superposition of cupin domains of dm CENP‐C and sp CENP‐C (Cnp3) (Chik et al, 2019). CENP‐C is shown orange and Cnp3 in silver. Dotted arrow highlights conformational changes in the loop regions.
- Calculated and experimentally determined molecular masses and sedimentation coefficients for plausible solution states of CAL1841–979, CENP‐C1264–1411 and complexes thereof.
- Structural superposition of CAL1 bound dm CENP‐C cupin domain onto sp CENP‐C cupin domain. dm CENP‐C is shown orange, sp CENP‐C in silver and CAL1 in blue. Amino acid residues identified to be crucial for Moa1 binding (by Chik et al, 2019) are shown in stick representation in green.
