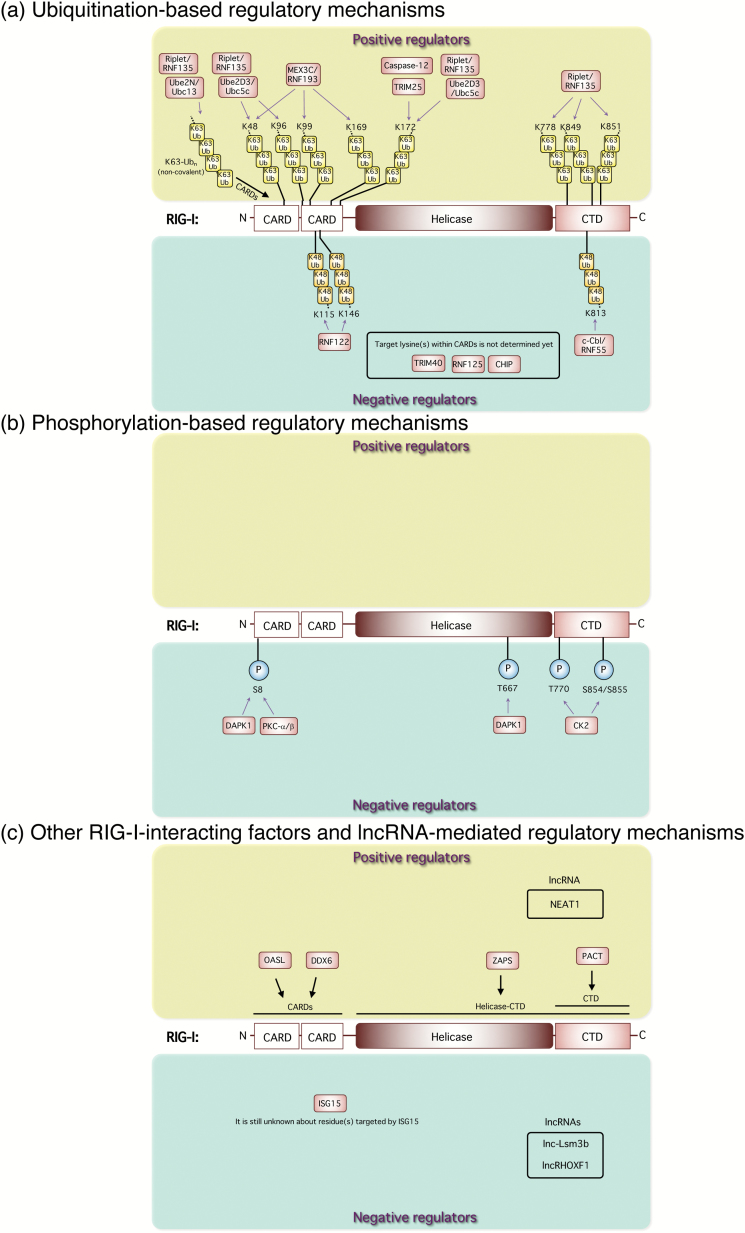
Fig. 2.
Regulatory mechanisms of RIG-I activation and its signaling. (a) Ubiquitination-based regulatory mechanisms. The RING-finger E3 Ub ligase TRIM25 positively mediates the RIG-I pathway by its K63-linked ubiquitination of the N-terminal CARDs of RIG-I. Riplet/RNF135 promotes K63-linked polyubiquitination of the C-terminal region of RIG-I. MEX3C/RNF193 mediates K63-linked polyubiquitination of the CARDs of RIG-I for its activation. Caspase-12 is physically associated with RIG-I and up-regulates TRIM25-mediated ubiquitination of RIG-I, leading to the enhancement of type I interferon responses. Non-covalent binding of K63-Ubn to the RIG-I CARDs induces its tetramer formation for downstream signal activation. Ube2D3/Ubc5c and Ube2N/Ubc13 were also identified as essential Ub-conjugating enzymes (E2) for RIG-I activation. The Ube2D3/Ubc5c–Riplet pair promotes covalent conjugation of polyubiquitin chains to RIG-I, whereas Ube2N/Ubc13 preferentially facilitates production of unanchored polyubiquitin chains. On the contrary, K48-linked ubiquitination of RIG-I by other E3 Ub ligases, such as c-Cbl/RNF55, RNF122, RNF125, CHIP and TRIM40, negatively regulates anti-viral innate responses. (b) Phosphorylation-based regulatory mechanisms. DAPK1 interacts with RIG-I and directly phosphorylates its threonine (T) 667 to impair its RNA ligand binding and abolishes anti-viral signaling. DAPK1 and PKC-α/β phosphorylate the N-terminal serine(S) at position 8 of RIG-I to suppress TRIM25-mediated RIG-I ubiquitination. CK2 is responsible for this regulation, and constitutively phosphorylates T770 and S854 to S855 residues in the RIG-I CTD, which keeps a closed, inactivated conformation. (c) Other RIG-I-interacting factors and lncRNA-mediated regulatory mechanisms. OASL interacts with RIG-I and mimics polyubiquitin through the UBL domains to enhance RIG-I-mediated anti-viral signaling. ISG15-mediated ISGylation of RIG-I reduces the cellular level of active RIG-I and suppresses its downstream anti-viral innate responses. ZAPS, which is a shorter form of PARP-13, functions as a potent stimulator of RIG-I-mediated anti-viral signaling through its interaction with RIG-I to facilitate RIG-I oligomerization and its ATPase activity for the activation of both IRF-3 and NF-κB. PACT directly interacts with the CTD of RIG-I to potentiate the activation of RIG-I. DDX6 interacts with RIG-I and augments type I interferon gene induction.