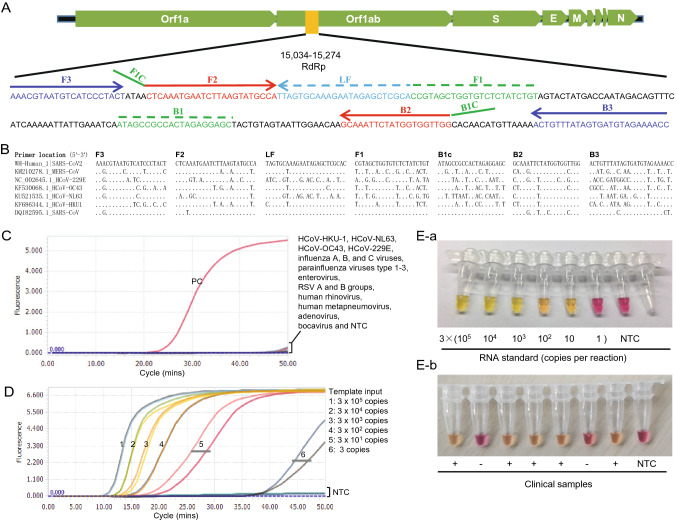
Fig. 1.
A Location of the primers in SARS-CoV-2 genome. B Sequence comparison among seven human coronaviruses (SARS-CoV2, SARS-CoV, MERS-CoV, OC43, HKU1, NL63 and 229E). C Cross-reactivity test of the novel SARS-CoV-2 RT-LAMP assay to other common respiratory viruses. Tested common respiratory viruses include HCoV-HKU-1, HCoV-NL63, HCoV-OC43, HCoV-229E, influenza A, B, and C viruses, parainfluenza viruses type 1–3, enterovirus, respiratory syncytial virus A and B groups, human rhinovirus, human metapneumovirus, adenovirus and bocavirus. RNA from a COVID-19 patient was used as positive control (PC). NTC, non-template control. D Sensitivity test of the novel SARS-CoV-2 RT-LAMP assay. Positive amplification was defined only when all three replicates are successfully amplified. NTC: non-template control. E Visual detection of SARS-CoV-2 by the colorimetric RT-LAMP assay. E-a RNA standards; E-b Clinical samples. NTC: non-template control.