Abstract
Antibodies against the protective antigen (PA) of Bacillus anthracis play a key role in response to infection by this important pathogen. The aim of this study was to produce and characterize monoclonal antibodies (mAbs) specific for PA and to identify novel neutralizing epitopes. Three murine mAbs with high specificity and nanomolar affinity for B. anthracis recombinant protective antigen (rPA) were produced and characterized. Western immunoblot analysis, coupled with epitope mapping using overlapping synthetic peptides, revealed that these mAbs recognize a linear epitope within domain 2 of rPA. Neutralization assays demonstrate that these mAbs effectively neutralize lethal toxin in vitro.
Keywords: anthrax, monoclonal antibody, neutralizing epitope, protective antigen
Introduction
The causative agent of anthrax, Bacillus anthracis, expresses three major extracellular toxin protein components, encoded on its large pXO1 plasmid (Okinaka et al., 1999). Protective antigen (PA) combines with either lethal factor (LF) or edema factor (EF) to form a functional binary toxin (reviewed in Abrami et al., 2005). PA binds to one of two cellular receptors, TEM8 (Bradley et al., 2001; Liu and Leppla, 2003) or CMG2 (Scobie et al., 2003). Upon receptor binding, the 83 kDa PA (PA83) is cleaved at a specific sequence by furin or a furin-like protease, releasing a 20 kDa N-terminal fragment (PA20) while leaving a 63 kDa C-terminal fragment (PA63) bound to the receptor (Singh et al., 1989; Molloy et al., 1992). An LF binding site on PA63 is simultaneously exposed via this cleavage event (Novak et al., 1992). Spontaneous heptamerization of the PA:receptor complex occurs (Milne et al., 1994), allowing competitive, high-affinity binding by EF or LF (Cunningham et al., 2002; Mogridge et al., 2002), followed by internalization of the toxin:receptor complex via clathrin-mediated endocytosis (Abrami et al., 2003). Acidification of the endosome produces structural rearrangements in the PA prepore heptamer, leading to pore formation and membrane insertion (Lacy et al., 2004; Santelli et al., 2004), and subsequent release of LF and/or EF into the cytosol (reviewed in Abrami et al., 2005).
PA is the primary antigenic determinant in currently licensed human anthrax vaccines (Turnbull et al., 1986; Leppla et al., 2002; Baillie et al., 2004; Adams et al., 2005). Several recent model studies demonstrate that a strong humoral response to PA contributes to a protective immune response to anthrax (Miller et al., 1998; Pitt et al., 2001; Reuveny et al., 2001; Little et al., 2004), and several regions that serve as targets for neutralizing monoclonal antibodies have been identified (Little et al., 1996; Brossier et al., 2004). The mature 735-amino-acid PA protein (GenBank accession number AAT98414) contains four distinct functional domains (Petosa et al., 1997). Domain 1 (residues 1–258) functions in oligomerization of PA and binding to LF and EF (Chauhan & Bhatnagar, 2002; Cunningham et al., 2002; Lacy et al., 2004), and contains the sequence 164RKKR167, which serves as the furin cleavage site (Singh et al., 1989; Molloy et al., 1992). Domain 2 (residues 259–487) functions in pore formation, heptamerization, membrane insertion, and translocation of EF and LF (Singh et al., 1994; Benson et al., 1998; Miller et al., 1999). Domain 3 (residues 488–595) functions in oligomerization (Mogridge et al., 2001), while domain 4 (residues 596–735) functions in binding the cellular receptor (Singh et al., 1991; Varughese et al., 1999; Santelli et al., 2004).
Multiple monoclonal antibodies (mAbs) that target different regions of PA and neutralize lethal toxin (LeTx) in vitro have been previously characterized. Several mAbs target epitopes in domain 4, and neutralize the toxin by preventing PA from binding to its cellular receptor (Little et al., 1988, 1996; Brossier et al., 2004). Other mAbs target epitopes in regions spanning the interfaces between domains 1 and 2 and domains 3 and 4, and prevent LF from interacting with PA at the cell surface (Little et al., 1996), or target epitopes in domain 2, preventing cleavage of PA83 to PA63 (Brossier et al., 2004). To identify unique neutralizing epitopes in PA, mAbs were raised in mice using whole recombinant protective antigen (rPA) as the immunogen, and their affinities and epitope specificities were characterized.
Materials and methods
Mouse immunization protocol and mAb production
For antibody production, pairs of 5- to 6-week-old BALB/c mice (Charles River, Wilmington, MA) were inoculated (day 1) subcutaneously with 5 µg of rPA (produced as described in Miller et al., 1999) in phosphate-buffered saline (PBS; pH 7.2), mixed with an equal volume of Complete Freund's Adjuvant (Difco, BD Biosciences, Oakville, ON, Canada). Subcutaneous boosters of 5 µg of rPA in PBS mixed with an equal portion of Incomplete Freund's Adjuvant (Difco) were given on days 30, 48 and 63. The mice were given a final intraperitoneal boost of 3 µg of rPA in PBS and sacrificed 3 days later. The rPA-specific humoral immune response was monitored via enzyme-linked immunosorbent assays (ELISA) using sera collected from the mice during the inoculation protocol, as described in Berry (2004), except the 96-well ELISA plates (MaxiSorp™, Nalge-NUNC, Rochester, NY) were coated with either rPA or, as a negative control, bovine serum albumin (BSA), both at 100 ng per well. Once sufficient anti-rPA titers were detected (OD405nm in ELISA at least three-fold above background), the mice were sacrificed, and hybridoma production and growth proceeded as described (Berry et al., 2004). mAb harvesting, concentration and isotyping were performed as described previously (Berry et al., 2004). Hybridoma supernatants were screened via the same ELISA to identify clones expressing high titers (OD405 in ELISA equal to or greater than that observed in the mouse immune serum) of rPA-specific mAbs. Mouse immune and preimmune sera (diluted 1 : 2000 with 0.2% BSA in PBS) served as positive and negative controls, respectively. The mAbs were purified using HiTrap™ Protein G HP columns according to the manufacturer's instructions (Amersham Biosciences, Uppsala, Sweden), the buffer was exchanged with PBS and the mAb concentrations were determined with a Micro BCA Protein Assay Kit according to the manufacturer's instructions (Pierce, Rockford, IL).
In vitro LeTx neutralization assays
LeTx neutralization was tested in vitro using the LeTx-sensitive mouse macrophage cell line J774A.1 (ATCC, Manassas, VA), essentially as described (Laffly et al., 2005). Briefly, the mouse macrophage adherent cell line J774A.1 was seeded at 105 cells per mL into the wells of a 96-well culture plate (96 Well Clear Flat Bottom Polystyrene TC-Treated Microplate, Corning, NY), and grown overnight in BD Cell™ mAb Medium, Quantum Yield (BD Biosciences, Bedford, MA) supplemented with 10% standard fetal bovine serum (HyClone, Logan, UT), 1%l-glutamine, and 1 × antibiotic–antimycotic solution (Wisent, St Bruno, QC, Canada) at 37°C in a 5% CO2 atmosphere. After overnight incubation the culture supernatant was removed from each well in the 96-well culture plate. In a separate 96-well ELISA plate, 100 µL aliquots of hybridoma supernatants (undiluted) or mAbs (diluted 1 : 10 to 1 : 100 000 in PBS) were added to appropriate wells. Into each test well containing diluted mAbs, 100 µL of a mixture of 2.0 µg mL−1 rPA and 1.0 µg mL−1 rLF (produced as described in Kassam et al., 2005), diluted in PBS, was added. Cell control wells contained PBS only, and did not receive any toxin or mAb, and toxin control wells contained toxin but no mAbs. This ELISA plate was then incubated at 37°C for 1 h. Aliquots (100 µL) of each dilution of each mAb containing toxin (or appropriate toxin or cell control) was then transferred from the ELISA plate into the wells of the 96-well culture plate containing the adherent J774A.1 cells. This plate was then incubated at 37°C for 2 h, whereupon 100 µL of fresh growth medium and 40 µL of CellTiter 96® AQueous One Solution Cell Proliferation Assay medium (Promega, Madison, WI) were added. The cells were incubated for a further 2–2.5 h at 37°C to allow for color development, and the plate scanned in an ELISA plate reader at 490 nm. In a second assay, appropriately diluted rPA was added to the J774A.1 cells in the absence of any mAb, and the cells were incubated for 1 h at 37°C. The mAbs were separately combined with rLF and incubated at 37°C for 1 h, and then applied to the cells to which the PA was already added. All component concentrations, dilutions, incubations and other relevant conditions in this second assay format were as outlined above. The lowest mAb dilution that resulted in an OD490 nm reading equal to 90% or greater of the no-toxin control was used to determine the neutralizing titer. All neutralization assays were performed at least in triplicate.
Endpoint ELISA determinations
rPA, rLF and BSA were diluted in PBS (pH 7.2) and each was coated at 100 ng per well in 96-well ELISA plates at 4°C overnight. The plates were then blocked with 10% skimmed milk in PBS for 90 min at 37°C, followed by three washes with 0.9% NaCl/0.05% Tween-20. mAbs were diluted (1 : 10 to 1 : 1010) in 2% BSA/PBS, applied to the wells and incubated at 37°C for 90 min. The wells were then washed four times, and incubated with the secondary antibody [horseradish peroxidase (HRP)-conjugated goat antimouse immunoglobulin G (IgG) F(ab′)2, Jackson ImmunoResearch, West Grove, PA) diluted 1 : 5000 in 2% BSA/PBS, at 37°C for 90 min. The wells were washed four times, and then color development was monitored for 15–60 min after the addition of 200 µL of ABTS developing solution (Roche Diagnostics, Indianapolis, IN), followed by scanning at 405 nm on an ELISA plate reader. All endpoint ELISA determinations were performed at least twice.
SDS-PAGE and Western immunoblotting
Immunoblots were performed essentially as described in Berry (2004). Briefly, 2 µg of rPA (or proteolytic digests thereof) were mixed with 10–20 µL of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (Bio-Rad, Hercules, CA), boiled for 5 min, and electrophoresed at 200 V for 1 h on a 4–20% Criterion Precast polyacrylamide gel (Bio-Rad) followed by electrophoretic transfer to nitrocellulose for 1 h at 100 V. Equal amounts of BSA and rLF were included as negative controls. Blocking proceeded for 1 h at room temperature in blocking buffer [10% skimmed milk in Tris-buffered saline/0.1% Tween-20 (TBST)]. mAbs were diluted 1 : 5000 in blocking buffer, and applied to the blots overnight at 4°C. The blots were washed with TBST as described (Berry et al., 2004), incubated at room temperature for 1 h with an HRP-conjugated goat antimouse IgG F(ab′)2 (Jackson Immuno Research) diluted 1 : 5000 with blocking buffer and finally washed as described (Berry et al., 2004). Development was performed using either 4-chloro-1-napthol substrate (Sigma, St Louis, MO) directly, or ECL Plus™ chemiluminescent detection reagent followed by exposure to Hyperfilm™ ECL™ (Amersham Biosciences, Piscataway, NJ), according to the manufacturer's instructions. Blots probed with rabbit serum were treated as described above, except that they were incubated with a 1 : 5000 dilution of immune serum from rabbits inoculated with rPA, and the secondary antibody used for detection was HRP-conjugated goat antirabbit IgG (Jackson ImmunoResearch). All immunoblots were performed in duplicate.
Pin-peptide epitope mapping
Peptides covering the entire length of PA were synthesized as 15-mers, overlapping by 10 residues, coupled to nylon support pins in a 96-well format (Pepscan Systems, Lelystad, the Netherlands). All manipulations of the pin-peptide assemblies were performed by placing the tips of the pins in the wells of ELISA plates (MaxiSorp™), ensuring they were fully submerged in the liquid samples. The pins were blocked with 200 µL of 4% BSA/PBS for 2 h at room temperature, followed by three washes with 0.9% NaCl/0.05% Tween 20 buffer. The pins were incubated with the mAbs (diluted to 1 : 500 or 1 : 1000 in 2% BSA/PBS) overnight at 4°C, and washed as described above. Incubation with 100 µL of HRP-conjugated goat antimouse IgG F(ab′)2 (Jackson ImmunoResearch) diluted 1 : 5000 in 2% BSA/PBS proceeded at room temperature for 4 h, followed by washing as described above. Color development and scanning was performed as described for the endpoint ELISAs above. The epitope mapping experiments were performed at least twice for each mAb. The cut-off value for positive binding was set at three times the average background OD405 nm value.
Proteolytic digestion of PA
Trypsin (TPCK-treated, from bovine pancreas, Sigma) was dissolved in 1 mM HCl, and α-chymotrypsin (Type VII, TLCK-treated, Sigma) was dissolved in 1 mM HCl/10 mM CaCl2 to make working stocks of 5 mg mL−1. Trypsin digests were performed in a total volume of 20 µL containing 2 µg of rPA mixed with 40 ng of trypsin. The digestion buffer was 100 mM Tris/HCl (pH 8). Chymotrypsin digests were performed identically, except the digestion buffer was 100 mM Tris/HCl (pH 8)/10 mM CaCl2. In both cases, the reactions were incubated on ice for 10 min, whereupon 2 µL of a 1 mg mL−1 solution of trypsin–chymotrypsin inhibitor (from soybean, Sigma) was added to stop the reactions. All proteolysis experiments were performed in duplicate.
mAb affinity analysis via surface plasmon resonance
Measurement of the affinity of the mAbs for rPA was performed essentially as described (Karlsson et al., 1991; Mabry et al., 2005) using a Biacore 2000 instrument (Biacore, Uppsala, Sweden). All solutions were purchased from Biacore. Briefly, a single flow cell on a CM5 sensor chip was activated by the addition of 20 µL of a 1 : 1 mixture of 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide hydrochloride:N-hydroxysuccinimide (EDC:NHS). Ten microliters of a 2.7 mg mL−1 solution of rPA was diluted in sodium acetate (pH 4), and 20 µL of this solution was coated on the activated chip. The chip was then blocked by the addition of 35 µL of ethanolamine-HCl, followed by a wash with 35 µL of 10 mM glycine-HCl (pH 1.5). The anti-PA mAbs were diluted in HBS-P buffer to final concentrations ranging from 889–2200 nM, and 40 µL of each dilution (five dilutions in total for each mAb) was applied in turn to the rPA-coated flow cell. The flow cell surface was regenerated in between additions of antibody dilutions via a wash with 35 µL of 10 mM glycine-HCl (pH 1.5). BIAevaluation 3.2 software was used to measure and plot the kon and koff values directly, which were then used to calculate the affinity (KD).
Results and discussion
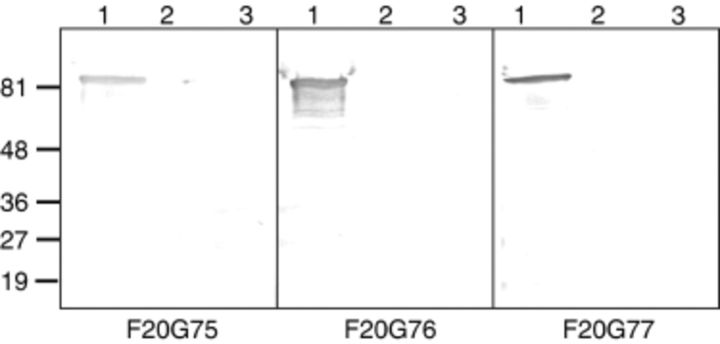
Eleven hybridoma clones expressing high titers of rPA-specific mAbs were identified, and all were of the IgG1/k isotype. After assaying all of the clones via an initial in vitro LeTx neutralization assay, three hybridoma clones, F20G75, F20G76 and F20G77, were chosen for further study. Sequencing of the expressed VH and VL region cDNAs indicates that these were distinct hybridomas formed by the immortalization of B cells with uniquely rearranged V genes (C. R. Corbett et al., unpublished data). To determine the nature of the rPA epitopes recognized by the anti-rPA mAbs, Western immunoblots were performed in duplicate; a representative immunoblot is shown in Fig. 1. All of the mAbs recognized an approximately 83 kDa protein that corresponds to mature rPA, and showed no cross-reactivity with either rLF or BSA. Binding to denatured protein suggests that these mAbs recognize a linear epitope (Cohen et al., 1986). The endpoint ELISA titer of the neutralizing mAbs specific for rPA (coated at 100 ng per well) was defined by the lowest mAb dilution resulting in a fivefold higher OD reading than the background reading obtained against BSA (Table 1). Specificity was good, with no significant reaction to rLF or BSA. Measurement of the affinity of the mAbs for rPA was performed via surface plasmon resonance analysis. As shown in Table 1, the KD for each mAb binding to rPA was in the nanomolar range. The kon value for each mAb was nearly identical, but F20G77 exhibited a significantly lower koff rate, which greatly increased its apparent affinity for rPA.
Figure 1.
Western immunoblot analysis probing specificity and monoclonal antibody (mAb) reactivity for recombinant protective antigen (rPA). Samples of 2 µg of protein were heat denatured and subjected to SDS-PAGE, followed by electrophoretic transfer to nitrocellulose membranes. The blots were probed with mAbs F20G75, F20G76 or F20G77, as indicated below each panel. Lane 1, rPA; lane 2, recombinant lethal factor (rLF); lane 3, bovine serum albumin. Protein size markers (kDa) are shown on the left of the figure.
Table 1.
Endpoint ELISA titers and affinity of the rPA-specific monoclonal antibodies (mAbs)
| Relevant properties | ||||
| mAb | Endpoint ELISA titer (ng mL−1) | k on for rPA binding (103 M−1 s−1) | k off for rPA binding (10−5 s−1) | Affinity (KD) for rPA binding (nM) |
| F20G75 | 20 | 3.4 ± 0.76 | 6.9 ± 0.40 | 20.8 ± 4.6 |
| F20G76 | 20 | 4.0 ± 1.2 | 6.8 ± 0.25 | 18.5 ± 5.9 |
| F20G77 | 20 | 3.1 ± 0.72 | 0.14 ± 0.013 | 0.46 ± 0.14 |
Average of three replicates.
Average of at least three replicates, ± SD.
To identify the rPA epitopes recognized by the mAbs, overlapping pin-peptides covering the entire sequence of PA were employed for epitope mapping. Each mAb reacted strongly to the same set of three overlapping 15-mers (Fig. 2), extending from S301 to S325. The core motif common to all three 15-mer peptides is 311ASFFD315. In the crystal structure of mature PA alone (Petosa et al., 1997), and in complex with receptor CMG2 (Santelli et al., 2004), the region of the 2β2–2β3 loop extending from H304 to S319, encompassing most of the above noted epitopes of the F20G75/76/77 mAbs, remains unresolved owing to its flexibility. Within the PA63 heptamer, this region undergoes structural rearrangements in the acidified endosome, leading to the production of a predicted extended β-barrel that spans the endosomal membrane (Petosa et al., 1997; Benson et al., 1998; Nassi et al., 2002; Santelli et al., 2004). Indeed, specific residues in the region extending from V303 to D315 (Qa'dan et al., 2005), including F313 and F314 (Singh et al., 1994; Benson et al., 1998), are involved in LF translocation, supporting the model that the 2β2–2β3 loop is involved in β-barrel formation. The 313FFD315 site in PA domain 2 is sensitive to chymotrypsin cleavage (Novak et al., 1992; Singh et al., 1994), suggesting that a portion of the flexible 2β2–2β3 loop containing these residues is solvent-exposed in the PA monomer (Singh et al., 1994). rPA was subjected to digestion by trypsin and chymotrypsin, and mAb binding to the proteolytic fragments was assayed using Western immunoblot. Trypsin cleaves PA at the 164RKKR167 sequence in domain 1, resulting in 63- and 20 kDa fragments (Novak et al., 1992), while chymotrypsin cleavage at the 313FFD315 sequence in domain 2 results in 47- and 37 kDa fragments (Singh et al., 1994). As shown in Fig. 3, trypsin cleavage had no effect on mAb recognition of the 63 kDa fragment of rPA, while chymotrypsin cleavage completely abrogated mAb binding, confirming that the epitope of all three mAbs extends across the 313FFD315 sequence in domain 2. This observation, coupled with the fact that three mAbs were developed that recognize the flexible 2β2–2β3 loop, lends support to the prediction that this region is exposed on the surface of PA.
Figure 2.
Protective antigen (PA) domain 2 peptide sequences recognized by monoclonal antibodies (mAbs) F20G75, F20G76 and F20G77, as determined by pin-peptide epitope mapping. Amino acid numbering is taken from the mature PA protein (GenBank accession number n). Residues common to all three peptides are in bold type.
Figure 3.
Western immunoblot analysis probing monoclonal antibody (mAb) reactivity with trypsin and chymotrypsin digests of recombinant protective antigen (rPA). Samples of 2 µg of rPA were subjected to digestion by 40 ng of trypsin or chymotrypsin for 10 min on ice, followed by the addition of inhibitor. The digests, and samples of 2 µg of undigested rPA, were then heat denatured and subjected to SDS-PAGE followed by electrophoretic transfer to nitrocellulose membranes. The blots were probed with mAbs F20G75 (a), F20G76 (b) or F20G77 (c). Lane 1, rPA; lane 2, bovine serum albumin; lane 3, trypsin-digested rPA; lane 4, chymotrypsin-digested rPA. To confirm that chymotrypsin digestion was effective, the same blots were washed and then reprobed with PA-specific rabbit polyclonal antiserum. A representative example of chymotrypsin-digested rPA probed with this antiserum is shown in (d). Size markers (kDa) are shown on the left of the figure.
To determine whether any particular residues in the identified PA domain 2 epitope were critical for mAb binding, a set of 15-mer pin-peptides was synthesized such that every residue extending from N306 to V320 was changed in turn to Ala (or in the case of existing Ala residues, to Gly). These peptides were assessed in the same manner as described for the epitope mapping employing overlapping peptides covering the whole PA sequence. As shown in Fig. 4, alteration of residues extending from A311 to D315 reduced mAb binding significantly, with F313A, F314A and D315A having the most apparent effect. The presence of two bulky, hydrophobic Phe residues in the middle of this epitope probably creates a specific peptide conformation that is critical for mAb recognition (Alvord et al., 1986; Warren et al., 1995). Interestingly, changing H310 to Ala increased apparent mAb binding efficiency by approximately twofold. The H310 residue in the epitope may constrain folding of the peptide via interaction with F314 or F315 (Yoshida et al., 2000), and, as opposed to the case of the F314A and F315A replacements, the H310A replacement might result in an alternate structural peptide conformation that leads to more efficient mAb binding.
Figure 4.
Pin-peptide epitope mapping to determine critical residues in the epitope extending from N306 to V320 in recombinant protective antigen (rPA). Synthetic peptides on a solid support matrix were synthesized such that every residue extending from N306 to V320 was altered in turn to Ala (or Gly in the case where an Ala was already present in the epitope) and their reactivities with monoclonal antibodies (mAbs) F20G75, F20G76 and F20G77 were assessed using ELISA. The background OD405 value was determined from reactivity of the mAbs with unrelated peptide sequences present on the same pin-peptide block. The OD reading for mAb binding to the ‘wild-type’ peptide (no changes to any amino acid within the epitope) was considered the baseline maximum binding level, to which the OD readings for mAbs binding to the altered peptides were compared as an indication of binding efficiency [% maximum OD=(OD of altered peptide/OD of unaltered peptide) × 100]. The assay was performed twice for each mAb, and the average value of two experiments is shown.
In vitro neutralization assays were employed to assay quantitatively the ability of the mAbs to neutralize LeTx. The neutralizing titers were determined by the lowest mAb concentration that resulted in an OD reading of at least 90–100% of that of the cell control samples (containing no toxin). Two formats of the same assay were employed. In the first, mAbs were coincubated with rPA and rLF prior to addition of the LeTx to the cells, whereas in the second, rPA was allowed to bind to the J774A.1 cells prior to addition of mAbs and rLF. Using the first assay format, the mAbs all neutralized LeTx, exhibiting neutralizing titers of 12.5 ng mL−1 (F20G75), 11.8 ng mL−1 (F20G76) and 16.0 ng mL−1 (F20G77). Another mAb, raised against a nonanthrax protein antigen, served as a negative control, and exhibited no neutralization activity. Interestingly, neutralization did not appear to be dose-responsive (Laffly et al., 2005; Brossier et al., 2004). Rather, neutralization appeared to be an ‘all or nothing’ event, with the ability of each mAb to neutralize LeTx remaining high at concentrations of 12–16 ng mL−1, until a dramatic decrease occurred once the mAbs were diluted to a concentration approaching 5–7 ng mL−1. This might be due to a strictly defined ‘threshold’ concentration of mAb molecules required to bind the specific epitope in rPA and inhibit LeTx activity. Once this minimal threshold level of mAb molecules is present in the local environment where PA, LF and the toxin receptor are present, LeTx activity is completely abrogated, and the presence of more mAbs in the environment causes no increase in neutralization. Alternatively, the high affinity of the neutralizing mAbs for PA might affect the dose responsiveness of the observed in vitro LeTx neutralization. As noted previously, an affinity-enhanced PA-specific neutralizing mAb (KD for PA binding of 0.33 nM) exhibited a much steeper LeTx neutralization dose–response curve compared with the parental mAb (KD for PA binding of 3.5–3.7 nM) from which it was derived (Mohamed et al., 2005). Similarly, a high-affinity (picomolar range) PA-specific neutralizing mAb lacking an Fc region exhibited a steep LeTx neutralization dose–response curve, although a different cell line was employed in that study (Mabry et al., 2005). Nevertheless, these reports do suggest that higher affinity anti-PA mAbs (or scAbs) can result in characteristically steep LeTx in vitro neutralization dose responsiveness. Using the second assay format, in which rPA was allowed to incubate with the J774A.1 macrophage cells prior to the addition of mAbs and rLF, some neutralization of LeTx was evident. However, mAb concentrations approaching 1–10 µg mL−1 were required for significant levels of neutralization to occur, and in some cases the OD readings in the presence of the mAbs in this second assay format only approached, but did not exceed, a level of 90% compared with the no-toxin controls. This observation indicates that neutralization was considerably more effective when the mAbs were able to bind to rPA prior to addition of the LeTx to the cells, and suggests that these mAbs cannot efficiently bind directly to rPA on the cell surface. Thus, it is probable that the mAbs do not act by blocking LF binding to surface-bound PA. In support of this observation, the H304-S319 ‘insertion loop’, which contains the epitope recognized by mAbs F20G75/76/77, is essentially buried between neighboring monomers in the heptameric prepore (Lacy et al., 2004), which would probably restrict mAb access to the epitope.
Several methods of neutralization can be envisaged for mAbs F20G75, F20G76 and F20G77. In one scenario, binding of the mAbs to the predicted surface-exposed epitope within the 2β2–2β3 loop of PA might result in regional conformational changes in PA that would prevent efficient receptor binding. An examination of the cocrystal structure of PA with CMG2 reveals that key interactions are made between the β3–β4 loop of domain 2 and CMG2 (Santelli et al., 2004), and because the 2β2–2β3 loop is in close proximity to the β3–β4 loop, binding of mAbs to the 2β2–2β3 loop might disrupt PA : receptor binding. Alternatively, binding of the mAbs to this region might create steric hindrance that either directly blocks access of PA to its receptor, or, more likely, prevents heptamerization after receptor binding. In this latter scenario, one can reason that mAb binding to the above noted epitope within the 2β2–2β3 loop region of PA could prevent the interaction of this domain with its nearest neighbor in the heptamer by creating a physical barrier to intersubunit binding. Regardless of which specific mechanism results in LeTx neutralization, it is clear from the data presented herein that these mAbs most likely neutralize LeTx at a step prior to the interaction of PA with its receptor and subsequent heptamer formation on the cell surface.
The data presented here suggest that domain 2 of PA is an immunogenic target for the development of LeTx-neutralizing mAbs, and that the 2β2–2β3 loop of domain 2 in rPA is solvent-accessible on the surface of the PA monomer. Coincidentally, the importance of amino acid residues 312SFFD315 within this region was recently confirmed using phage peptide display techniques (Zhang et al., 2006). The observations summarized herein will aid in the development of immunodiagnostic reagents and subunit vaccine candidates for the detection and treatment of B. anthracis infection.
Acknowledgements
This work was supported by funding for projects CRTI 03-0021TD and CRTI 0091RD, from the CBRN Research and Technology Initiative (CRTI). Thanks are extended to the staff of the RDU and ADAPT units at the National Microbiology Laboratory for their expert technical help.
References
- Abrami L., Liu S., Cosson P., Leppla S.H., Van Der Goot F.G. (2003) Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol 160: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L., Reig N., Van Der Goot F.G. (2005) Anthrax toxin: the long and winding road that leads to the kill. Trends Microbiol 13: 72–78. [DOI] [PubMed] [Google Scholar]
- Adams T., Osborn S., Rijpkema S. (2005) An immuno-diffusion assay to assess the protective antigen content of anthrax vaccine. Vaccine 23: 4517–4520. [DOI] [PubMed] [Google Scholar]
- Alvord E.C., Jr, Hruby S., Martenson R.E., Deibler G.E., Law M.J. (1986) Evidence for specific polypeptide chain folding in myelin basic protein from reactions between fragments of the protein and monoclonal antibodies. J Neurochem 47: 764–771. [DOI] [PubMed] [Google Scholar]
- Baillie L., Townend T., Walker N., Eriksson U., Williamson D. (2004) Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol Med Microbiol 42: 267–270. [DOI] [PubMed] [Google Scholar]
- Benson E.L., Huynh P.D., Finkelstein A., Collier R.J. (1998) Identification of residues lining the anthrax protective antigen channel. Biochemistry 37: 3941–3948. [DOI] [PubMed] [Google Scholar]
- Berry J.D., Jones S., Drebot M.A., et al. (2004) Development and characterisation of neutralising monoclonal antibody to the SARS-coronavirus. J Virol Methods 120: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K.A., Mogridge J., Mourez M., Collier R.J., Young J.A. (2001) Identification of the cellular receptor for anthrax toxin. Nature 414: 225–229. [DOI] [PubMed] [Google Scholar]
- Brossier F., Levy M., Landier A., Lafaye P., Mock M. (2004) Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect Immun 72: 6313–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V., Bhatnagar R. (2002) Identification of amino acid residues of anthrax protective antigen involved in binding with lethal factor. Infect Immun 70: 4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G.H., Isola V.J., Kuhns J., Berman P.W., Eisenberg R.J. (1986) Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with western blotting. J Virol 60: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K., Lacy D.B., Mogridge J., Collier R.J. (2002) Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc Natl Acad Sci USA 99: 7049–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson R., Michaelsson A., Mattsson L. (1991) Kinetic analysis of monoclonal antibody–antigen interactions with a new biosensor based analytical system. J Immunol Methods 145: 229–240. [DOI] [PubMed] [Google Scholar]
- Kassam A., Der S.D., Mogridge J. (2005) Differentiation of human monocytic cell lines confers susceptibility to Bacillus anthracis lethal toxin. Cell Microbiol 7: 281–292. [DOI] [PubMed] [Google Scholar]
- Lacy D.B., Wigelsworth D.J., Melnyk R.A., Harrison S.C., Collier R.J. (2004) Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc Natl Acad Sci USA 101: 13147–13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffly E., Danjou L., Condemine F., Vidal D., Drouet E., Lefranc M.P., Bottex C., Thullier P. (2005) Selection of a macaque Fab with framework regions like those in humans, high affinity, and ability to neutralize the protective antigen (PA) of Bacillus anthracis by binding to the segment of PA between residues 686 and 694. Antimicrob Agents Chemother 49: 3414–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S.H., Robbins J.B., Schneerson R., Shiloach J. (2002) Development of an improved vaccine for anthrax. J Clin Invest 110: 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S.F., Leppla S.H., Cora E. (1988) Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect Immun 56: 1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S.F., Novak J.M., Lowe J.R., Leppla S.H., Singh Y., Klimpel K.R., Lidgerding B.C., Friedlander A.M. (1996) Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142: 707–715. [DOI] [PubMed] [Google Scholar]
- Little S.F., Ivins B.E., Fellows P.F., Pitt M.L., Norris S.L., Andrews G.P. (2004) Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22: 422–430. [DOI] [PubMed] [Google Scholar]
- Liu S., Leppla S.H. (2003) Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem 278: 5227–5234. [DOI] [PubMed] [Google Scholar]
- Mabry R., Rani M., Geiger R., Hubbard G.B., Carrion R., Jr, Brasky K., Patterson J.L., Georgiou G., Iverson B.L. (2005) Passive protection against anthrax by using a high-affinity antitoxin antibody fragment lacking an Fc region. Infect Immun 73: 8362–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McBride B.W., Manchee R.J., Moore P., Baillie L.W. (1998) Production and purification of recombinant protective antigen and protective efficacy against Bacillus anthracis. Lett Appl Microbiol 26: 56–60. [DOI] [PubMed] [Google Scholar]
- Miller C.J., Elliott J.L., Collier R.J. (1999) Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38: 10432–10441. [DOI] [PubMed] [Google Scholar]
- Milne J.C., Furlong D., Hanna P.C., Wall J.S., Collier R.J. (1994) Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J Biol Chem 269: 20607–20612. [PubMed] [Google Scholar]
- Mogridge J., Mourez M., Collier R.J. (2001) Involvement of domain 3 in oligomerization by the protective antigen moiety of anthrax toxin. J Bacteriol 183: 2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogridge J., Cunningham K., Lacy D.B., Mourez M., Collier R.J. (2002) The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc Natl Acad Sci USA 99: 7045–7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N., Clagett M., Li J., Jones S., Pincus S., D'Alia G., Nardone L., Babin M., Spitalny G., Casey L. (2005) A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect Immun 73: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy S.S., Bresnahan P.A., Leppla S.H., Klimpel K.R., Thomas G. (1992) Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem 267: 16396–16402. [PubMed] [Google Scholar]
- Nassi S., Collier R.J., Finkelstein A. (2002) PA63 channel of anthrax toxin: an extended beta-barrel. Biochemistry 41: 1445–1450. [DOI] [PubMed] [Google Scholar]
- Novak J.M., Stein M.P., Little S.F., Leppla S.H., Friedlander A.M. (1992) Functional characterization of protease-treated Bacillus anthracis protective antigen. J Biol Chem 267: 17186–17193. [PubMed] [Google Scholar]
- Okinaka R.T., Cloud K., Hampton O., et al. (1999) Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J Bacteriol 181: 6509–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petosa C., Collier R.J., Klimpel K.R., Leppla S.H., Liddington R.C. (1997) Crystal structure of the anthrax toxin protective antigen. Nature 385: 833–838. [DOI] [PubMed] [Google Scholar]
- Pitt M.L., Little S.F., Ivins B.E., Fellows P., Barth J., Hewetson J., Gibbs P., Dertzbaugh M., Friedlander A.M. (2001) In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19: 4768–4773. [DOI] [PubMed] [Google Scholar]
- Qa'dan M., Christensen K.A., Zhang L., Roberts T.M., Collier R.J. (2005) Membrane insertion by anthrax protective antigen in cultured cells. Mol Cell Biol 25: 5492–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny S., White M.D., Adar Y.Y., Kafri Y., Altboum Z., Gozes Y., Kobiler D., Shafferman A., Velan B. (2001) Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun 69: 2888–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelli E., Bankston L.A., Leppla S.H., Liddington R.C. (2004) Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature 430: 905–908. [DOI] [PubMed] [Google Scholar]
- Scobie H.M., Rainey G.J., Bradley K.A., Young J.A. (2003) Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA 100: 5170–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y., Chaudhary V.K., Leppla S.H. (1989) A deleted variant of Bacillus anthracis protective antigen is non-toxic and blocks anthrax toxin action in vivo. J Biol Chem 264: 19103–19107. [PubMed] [Google Scholar]
- Singh Y., Klimpel K.R., Quinn C.P., Chaudhary V.K., Leppla S.H. (1991) The carboxyl-terminal end of protective antigen is required for receptor binding and anthrax toxin activity. J Biol Chem 266: 15493–15497. [PubMed] [Google Scholar]
- Singh Y., Klimpel K.R., Arora N., Sharma M., Leppla S.H. (1994) The chymotrypsin-sensitive site, FFD315, in anthrax toxin protective antigen is required for translocation of lethal factor. J Biol Chem 269: 29039–29046. [PubMed] [Google Scholar]
- Turnbull P.C., Broster M.G., Carman J.A., Manchee R.J., Melling J. (1986) Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect Immun 52: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varughese M., Teixeira A.V., Liu S., Leppla S.H. (1999) Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect Immun 67: 1860–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K.G., Catz I., Steinman L. (1995) Fine specificity of the antibody response to myelin basic protein in the central nervous system in multiple sclerosis: the minimal B-cell epitope and a model of its features. Proc Natl Acad Sci USA 92: 11061–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Matsushima N., Kumaki Y., Nakata M., Hikichi K. (2000) NMR studies of model peptides of PHGGGWGQ repeats within the N-terminus of prion proteins: a loop conformation with histidine and tryptophan in close proximity. J Biochem (Tokyo) 128: 271–281. [DOI] [PubMed] [Google Scholar]
- Zhang J., Xu J., Li G., Dong D., Song X., Guo Q., Zhao J., Fu L., Chen W. (2006) The 2β2–2β3 loop of anthrax protective antigen contains a dominant neutralizing epitope. Biochem Biophys Res Commun 341: 1164–1171. [DOI] [PubMed] [Google Scholar]