Figure 6.
Topological Comparison of the M48USP Structure to Other Cysteine Proteases and Deubiquitinating Enzymes
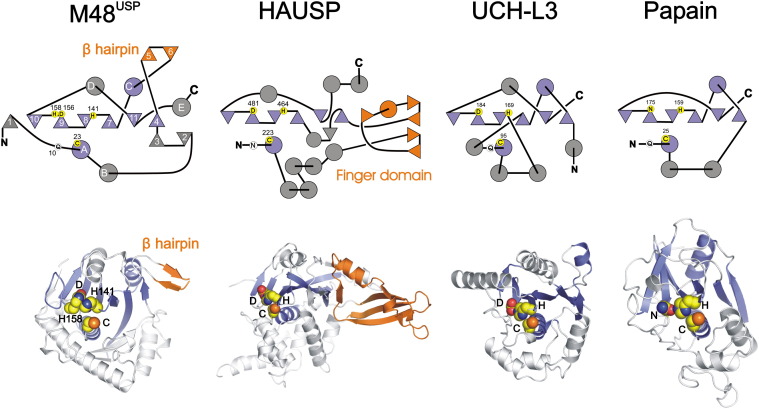
The topographies (top) and ribbon diagrams (bottom) of M48USP and representatives of the USP (HAUSP) and UCH family (UCH-L3) of DUBs, and papain, are shown. Circles and triangles represent α helices and β sheets, respectively. In the topology diagram of M48USP, secondary structure elements are numbered sequentially according to Figure 2A. Secondary structure elements conserved within the cysteine protease superfamily, exemplified by papain, are shown in blue. The exposed β hairpin of M48USP and the finger domain of HAUSP are orange; both interact with the Ub core. Catalytic triad residues are in yellow circles, whereas oxyanion hole residues are in white circles. Note that the sequential order of the more efficient M48USP catalytic triad (C23, D156, and H158) is different compared to other cysteine proteases and that M48USP shares more similarities to the papain topography than to the two DUB representatives.