Table 1.
The physicochemical, elemental and spectral characteristics of synthesized thiazolidine-2,4-dione derivatives
| Compound | Physicochemical and spectral characteristics |
|---|---|
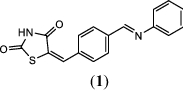 |
5-((E)-4-((E)-(phenylimino)methyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 260-262; Rf value: 0.60b; % yield: 80; IR (KBr pellets) cm−1: 3375.41 (N–H str., thiazolidine ring), 1742.41 (C=O str., thiazolidin-2,4-dione ring), 1696.11 (C=N str., imine group), 1414.1 (C=C str., aromatic ring), 1602.13 (C=C str., methylene group), 3044.78 (C–H str., aromatic ring), 2919.12 (C–H str., aliphatic), 1317.75 (C–N str., thiazolidine ring), 614.77 (C–S bend., thiazolidine ring); 1H-NMR (δ, DMSO): 6.52–7.75 (m, 9H, Ar–H), 7.77 (s, 1H, –CH=), 8.71(s, 1H, CH=N), 12.49 (s, 1H, NH); M. Formula: C17H12N2O2S; MS: m/z 308 (M+); Elemental analysis (CHN) Theoretical calc: C, 66.22; H, 3.92; N, 9.08 Found: C, 66.25; H, 3.90; N, 9.09 |
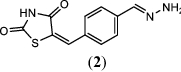 |
5-((E)-4-((E)-hydrazonomethyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 220–222; Rf value: 0.74c; % yield: 60; IR (KBr pellets) cm−1: 1740.11 (C=O str., thiazolidin-2,4-dione ring), 1682.74 (C=N str., imine group), 1412.68 (C=C str., aromatic ring), 1615.22 (C=C str., methylene group), 3197.24 (C–H str., aromatic ring), 2922.17 (C–H str., aliphatic), 1224.86 (C–N str., thiazolidine ring), 618.11 (C–S bend., thiazolidine ring); 1H-NMR (δ, DMSO): 6.58–7.79 (m, 4H, Ar–H), 7.81 (s, 1H, –CH=), 8.68(s, 1H, CH=N), 1.92 (s, 2H, NH2), 12.49 (s, 1H, NH); M. Formula: C11H9N3O2S; Elemental analysis (CHN) Theoretical calc: C, 53.43; H, 3.67; N, 16.99 Found: C, 53.45; H, 3.68; N, 16.95 |
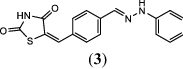 |
5-((E)-4-((E)-(2-phenylhydrazono)methyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 308–310; Rf value: 0.8d; % yield: 78; IR (KBr pellets) cm−1: 3441.91 (N–H str., phenyl hydrazine group), 3286.35 (N–H str., thiazolidine ring), 1726.16 (C=O str., thiazolidin-2,4-dione ring), 1677.76 (C=N str., imine group), 1413.66 (C=C str., aromatic ring), 1584.3 (C=C str., methylene group), 3043.30 (C–H str., aromatic ring), 1328.68 (C–N str., thiazolidine ring), 613.72 (C–S bend., thiazolidine ring); 1H-NMR (δ, DMSO): 6.77–7.79 (m, 4H, Ar–H), 7.80 (s, 1H, –CH=), 7.88(s, 1H, CH=N), 10.61 (s, 1H, NH adjacent to phenyl ring), 12.49 (s, 1H, NH of thiazolidine ring); M. Formula: C17H13N3O2S; MS: m/z 323.9 (M+ + 1), 321.9 (M+ − 1); Elemental analysis (CHN) Theoretical calc: C, 63.14; H, 4.05; N, 12.99 Found: C, 63.16; H, 4.05; N, 12.98 |
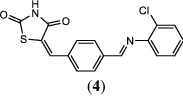 |
5-((E)-4-((E)-((2-chlorophenyl)imino)methyl)benzylidene)thiazolidine -2,4-dione: m.p. °C: 292–294; Rf value: 0.71d; % yield: 70; IR (KBr pellets) cm−1: 3381.37 (N–H str., thiazolidine ring), 1741.97 (C=O str., thiazolidin-2,4-dione ring), 1694.84 (C=N str., imine group), 1505.9 (C=C str., aromatic ring), 1606.57 (C=C str., methylene group), 3044.79 (C–H str., aromatic ring), 2978.2 (C–H str., aliphatic), 1305.25 (C–N str., thiazolidine ring), 606.87 (C–S bend., thiazolidine ring), 750.70 (C–Cl bend., o-substitution on phenyl ring); 1H NMR (δ, DMSO): 6.77–7.79 (m, 4H, Ar–H), 7.80 (s, 1H, –CH=), 7.88(s, 1H, CH=N), 10.61 (s, 1H, NH adjacent to phenyl ring), 12.49 (s, 1H, NH of thiazolidine ring); M. Formula: C17H11ClN2O2S; Elemental analysis (CHN) Theoretical calc: C, 59.56; H, 3.23; N, 8.17 Found: C, 59.60; H, 3.25; N, 8.12 |
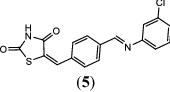 |
5-((E)-4-((E)-((3-chlorophenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 280–282; Rf value: 0.73d; % yield: 75; IR (KBr pellets) cm−1: 3381.59 (N–H str., thiazolidine ring), 1741.81 (C=O str., thiazolidin-2,4-dione ring), 1695.1 (C=N str., imine group), 1479.44 (C=C str., aromatic ring), 1596.41 (C=C str., methylene group), 3050.78 (C–H str., aromatic ring), 2978.82 (C–H str., aliphatic), 1308.79 (C–N str., thiazolidine ring), 594.24 (C–S bend., thiazolidine ring), 777.34 (C–Cl bend., m-substitution on phenyl ring); 1H NMR (δ, DMSO): 7.23–8.07 (m, 8H, Ar–H), 7.81 (s, 1H, –CH=), 8.68 (s, 1H, CH=N), 12.58 (s, 1H, NH); M. Formula: C17H11ClN2O2S; MS: m/z 341.43 (M+ − 1); Elemental analysis (CHN) Theoretical calc: C, 59.56; H, 3.23; N, 8.17 Found: C, 59.62; H, 3.23; N, 8.18 |
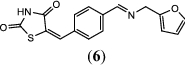 |
5-((E)-4-((E)-((furan-2-ylmethyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 92–94; Rf value: 0.71a; % yield: 70; IR IR (KBr pellets) cm−1: 3328.11 (N–H str., thiazolidine ring), 1694.77 (C=O str., thiazolidin-2,4-dione ring), 1610.48 (C=N str., imine group), 1423.16 (C=C str., aromatic ring), 1548.31 (C=C str., methylene group), 3045.54 (C–H str., aromatic ring), 2975.08 (C–H str., aliphatic), 1306.66 (C–N str., thiazolidine ring), 603.55 (C–S bend., thiazolidine ring), 1159.90 (C–C str.), 1012.66 (C–O–C str., furan ring); 1H NMR (δ, DMSO): 7.18–7.88 (m, 4H, Ar–H), 7.78 (s, 1H, –CH=), 8.76 (s, 1H, CH=N), 12.48 (s, 1H, NH), 4.77 (d, 2H, –CH2 adjacent to furan ring), 6.30 (d, 1H, CH of furan ring at 3rd position), 6.42 (t, 1H, CH of furan ring at 4th position), 7.47 (d, 1H, CH of furan ring adjacent to O); M. Formula: C16H12N2O3S; MS: m/z 313.16 (M+ + 1); Elemental analysis (CHN) Theoretical calc: C, 61.53; H, 3.87; N, 8.97 Found: C, 61.55; H, 3.90; N, 8.95 |
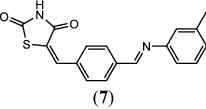 |
5-((E)-4-((E)-(m-tolylimino)methyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 90–92; Rf value: 0.81a; % yield: 65; IR (KBr pellets) cm−1: 3378.54 (N–H str., thiazolidine ring), 1743.48 (C=O str., thiazolidin-2,4-dione ring), 1691.85 (C=N str., imine group), 1487.15 (C=C str., aromatic ring), 1602.26 (C=C str., methylene group), 3036.12 (C–H str., aromatic ring), 2967.46 (C–H str., aliphatic), 1321.95 (C–N str., thiazolidine ring), 597.53 (C–S bend., thiazolidine ring), 1154.43 (C–C str.); 1H NMR (δ, DMSO): 7.08–8.06 (m, 8H, Ar–H), 7.62 (s, 1H, –CH=), 8.43 (s, 1H, CH=N), 12.34 (s, 1H, NH), 2.14(s, 3H, CH3); M. Formula: C18H14N2O2S; MS: m/z 320.96 (M+ − 1); Elemental analysis (CHN) Theoretical calc: C, 67.06; H, 4.38; N, 8.69 Found: C, 67.09; H, 4.39; N, 8.71 |
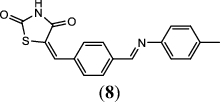 |
5-((E)-4-((E)-(p-tolylimino)methyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 160–162; Rf value: 0.84a; % yield: 75; IR (KBr pellets) cm−1: 3375.37 (N–H str., thiazolidine ring), 1744.26 (C=O str., thiazolidin-2,4-dione ring), 1695.41 (C=N str., imine group), 1507.28 (C=C str., aromatic ring), 1604.31 (C=C str., methylene group), 3162.34 (C–H str., aromatic ring), 3048.39 (C–H str., aliphatic), 1330.13 (C–N str., thiazolidine ring), 607.48 (C–S bend., thiazolidine ring), 1155.49 (C–C str.); 1H NMR (δ, DMSO): 6.80–8.06 (m, 8H, Ar–H), 7.72 (s, 1H, –CH=), 8.71 (s, 1H, CH=N), 12.32 (s, 1H, NH), 2.33(s, 3H, CH3); M. Formula: C18H14N2O2S; MS: m/z 322.01 (M+); Elemental analysis (CHN) Theoretical calc: C, 67.06; H, 4.38; N, 8.69 Found: C, 67.11; H, 4.39; N, 8.65 |
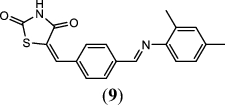 |
5-((E)-4-((E)-((2,4-dimethylphenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 134–136; Rf value: 0.81a; % yield: 65; IR (KBr cm−1): 3398.47 (N–H str., thiazolidine ring), 1744.74 (C=O str., thiazolidin-2,4-dione ring), 1699.13 (C=N str., imine group), 1498.40 (C=C str., aromatic ring), 1601.47 (C=C str., methylene group), 3012.68 (C–H str., aromatic ring), 2973.04 (C–H str., aliphatic), 1291.9 (C–N str., thiazolidine ring), 605.41 (C–S bend., thiazolidine ring), 1152.01 (C–C str.); 1H NMR (δ, DMSO): 7.02–8.06 (m, 7H, Ar–H), 7.82 (s, 1H, –CH=), 8.58 (s, 1H, CH=N), 12.12 (s, 1H, NH), 2.28(s, 3H, CH3 o-position), 2.29(s, 3H, CH3 p-position); M. Formula: C19H16N2O2S; MS: m/z 334.99 (M+ − 1); Elemental analysis (CHN) Theoretical calc: C, 67.84; H, 4.79; N, 8.33 Found: C, 67.87; H, 4.79; N, 8.36 |
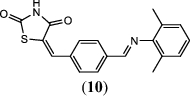 |
5-((E)-4-((E)-((2,6-dimethylphenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 100–102; Rf value: 0.73a; % yield: 60; IR (KBr cm−1): 3394.22 (N–H str., thiazolidine ring), 1744.97 (C=O str., thiazolidin-2,4-dione ring), 1702.53 (C=N str., imine group), 1470.88 (C=C str., aromatic ring), 1602.92 (C=C str., methylene group), 3032.72 (C–H str., aromatic ring), 2971.09 (C–H str., aliphatic), 1294.35 (C–N str., thiazolidine ring), 606.56 (C–S bend., thiazolidine ring), 1159.9 (C–C str.); 1H NMR (δ, DMSO): 6.77–8.10 (m, 7H, Ar–H), 7.84 (s, 1H, –CH=), 8.42 (s, 1H, CH=N), 12.32 (s, 1H, NH), 2.08(s, 6H, CH3 o-position); M. Formula: C19H16N2O2S; MS: m/z 334.98 (M+ − 1), 337 (M+ + 1); Elemental analysis (CHN) Theoretical calc: C, 67.84; H, 4.79; N, 8.33 Found: C, 67.85; H, 4.79; N, 8.36 |
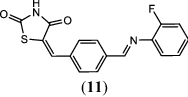 |
5-((E)-4-((E)-((2-fluorophenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 108–110; Rf value: 0.69a; % yield: 75; IR (KBr cm−1): 3429.95 (N–H str., thiazolidine ring), 1745.15 (C=O str., thiazolidin-2,4-dione ring), 1695.31 (C=N str., imine group), 1492.16 (C=C str., aromatic ring), 1604.74 (C=C str., methylene group), 3051.62 (C–H str., aromatic ring), 2979.31 (C–H str., aliphatic), 1323.63 (C–N str., thiazolidine ring), 606.47 (C–S bend., thiazolidine ring), 1158.78 (C–C str.), 1009.17 (C-F bend., o-substitution on phenyl ring); 1H NMR (δ, DMSO): 7.26–7.76 (m, 8H, Ar–H), 7.83 (s, 1H, –CH=), 8.68 (s, 1H, CH=N), 12.51 (s, 1H, NH); M. Formula: C17H11N2O2SF; MS: m/z 327.05 (M+ + 1); Elemental analysis (CHN) Theoretical calc: C, 62.57; H, 3.40; N, 8.58 Found: C, 62.60; H, 3.41; N, 8.60 |
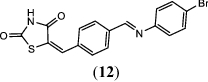 |
5-((E)-4-((E)-((3-bromophenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 98–100; Rf value: 0.82a; % yield: 80; IR (KBr cm−1): 3437.95 (N–H str., thiazolidine ring), 1744.75 (C=O str., thiazolidin-2,4-dione ring), 1698.27 (C=N str., imine group), 1484.71 (C=C str., aromatic ring), 1606.87 (C=C str., methylene group), 3052.46 (C–H str., aromatic ring), 2976.98 (C–H str., aliphatic), 1328.18 (C–N str., thiazolidine ring), 606.38 (C–S bend., thiazolidine ring), 1161.85 (C–C str.), 637.54 (C–Br bend., p-substitution on phenyl ring); 1H NMR (δ, DMSO): 7.03–8.12 (m, 8H, Ar–H), 7.78 (s, 1H, –CH=), 8.72 (s, 1H, CH=N), 12.64 (s, 1H, NH); M. Formula: C17H11N2O2SBr; Elemental analysis (CHN) Theoretical calc: C, 52.73; H, 2.86; N, 7.23 Found: C, 52.75; H, 2.87; N, 7.23 |
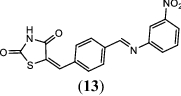 |
5-((E)-4-((E)-((3-nitrophenyl)imino)methyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 128–130; Rf value: 0.79a; % yield: 80; IR (KBr cm−1): 3379.48 (N–H str., thiazolidine ring), 1747.82 (C=O str., thiazolidin-2,4-dione ring), 1700.55 (C=N str., imine group), 1522.68 (C=C str., aromatic ring), 1600.01 (C=C str., methylene group), 3034.11 (C–H str., aromatic ring), 2928.16 (C–H str., aliphatic), 1347.29 (C–N str., thiazolidine ring), 606.91 (C–S bend., thiazolidine ring), 1153.91 (C–C str.), 1208.69 (N–O str., m-substitution on phenyl ring), 1413.00 (N=O str., m-substitution on phenyl ring); 1H NMR (δ, DMSO): 7.23–8.09 (m, 8H, Ar–H), 7.77 (s, 1H, –CH=), 8.80 (s, 1H, CH=N), 12.67 (s, 1H, NH); M. Formula: C17H11N3O4S; MS: m/z 352.04 (M+ − 1); Elemental analysis (CHN) Theoretical calc: C, 57.79; H, 3.14; N, 11.89 Found: C, 57.82; H, 3.15; N, 11.91 |
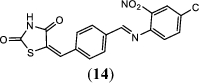 |
5-((E)-4-((E)-((4-chloro-2-nitrophenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 92–94; Rf value: 0.81a; % yield: 75; IR (KBr cm−1): 3356. 19 (N–H str., thiazolidine ring), 1747.59 (C=O str., thiazolidin-2,4-dione ring), 1700.69 (C=N str., imine group), 1502.15 (C=C str., aromatic ring), 1602.45 (C=C str., methylene group), 3030.51 (C–H str., aromatic ring), 2926.60 (C–H str., aliphatic), 1338.08 (C–N str., thiazolidine ring), 607.48 (C–S bend., thiazolidine ring), 1160.38 (C–C str.), 1249.21 (N–O str., o-substitution on phenyl ring), 1456.19 (N=O str., o-substitution on phenyl ring), 764.11 (C–Cl bend., p-substitution on phenyl ring); 1H NMR (δ, DMSO): 7.03–8.01 (m, 7H, Ar–H), 7.73 (s, 1H, –CH=), 8.03 (s, 1H, CH=N), 12.70 (s, 1H, NH); M. Formula: C17H10N3O4SCl; Elemental analysis (CHN) Theoretical calc: C, 52.65; H, 2.60; N, 10.84 Found: C, 52.67; H, 2.60; N, 10.87 |
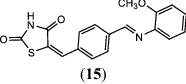 |
5-((E)-4-((E)-((2-methoxyphenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 104–106; Rf value: 0.81a; % yield: 70; IR (KBr cm−1): 3396.28 (N–H str., thiazolidine ring), 1744.31 (C=O str., thiazolidin-2,4-dione ring), 1702.51 (C=N str., imine group), 1504.09 (C=C str., aromatic ring), 1602.96 (C=C str., methylene group), 3050.93 (C–H str., aromatic ring), 2930.71 (C–H str., aliphatic), 1293.57 (C–N str., thiazolidine ring), 606.45 (C–S bend., thiazolidine ring), 1161.16 (C–C str.), 1018.82 (O–CH3 str., o-substitution on phenyl ring); 1H NMR (δ, DMSO): 6.75–7.72 (m, 8H, Ar–H), 7.78 (s, 1H, –CH=), 8.58 (s, 1H, CH=N), 12.61 (s, 1H, NH), 3.77 (s, 3H, OCH3); M. Formula: C18H14N2O3S; MS: m/z 339.12 (M+ + 1); Elemental analysis (CHN) Theoretical calc: C, 63.89; H, 4.17; N, 8.28 Found: C, 63.91; H, 4.17; N, 8.33 |
 |
5-((E)-4-((E)-((3-methoxyphenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 176–178; Rf value: 0.82a; % yield: 65; IR (KBr cm−1): 3380.83 (N–H str., thiazolidine ring), 1742.00 (C=O str., thiazolidin-2,4-dione ring), 1696.51 (C=N str., imine group), 1498.71 (C=C str., aromatic ring), 1601.06 (C=C str., methylene group), 2926.44 (C–H str., aliphatic), 1321.31 (C–N str., thiazolidine ring), 605.30 (C–S bend., thiazolidine ring), 1155.65 (C–C str.), 1029.32 (O–CH3 str., m-substitution on phenyl ring); 1H NMR (δ, DMSO): 6.58–8.05 (m, 8H, Ar–H), 7.73 (s, 1H, –CH=), 8.68 (s, 1H, CH=N), 12.32 (s, 1H, NH), 3.85(s, 3H, OCH3); M. Formula: C18H14N2O3S; MS: m/z 338.8 (M+ + 1); Elemental analysis (CHN) Theoretical calc: C, 63.89; H, 4.17; N, 8.28 Found: C, 63.92; H, 4.17; N, 8.31 |
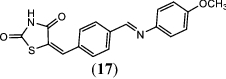 |
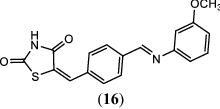
5-((E)-4-((E)-((4-methoxyphenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 130–132; Rf value: 0.77a; % yield: 75; IR (KBr cm−1): 3444.26 (N–H str., thiazolidine ring), 1743.88 (C=O str., thiazolidin-2,4-dione ring), 1702.79 (C=N str., imine group), 1505.14 (C=C str., aromatic ring), 1614.33 (C=C str., methylene group), 3065.92 (C–H str., aromatic ring), 2926.15 (C–H str., aliphatic), 1291.79 (C–N str., thiazolidine ring), 606.22 (C–S bend., thiazolidine ring), 1155.22 (C–C str.), 1024.26 (O–CH3 str., o-substitution on phenyl ring); 1H NMR (δ, DMSO): 6.98–8.04 (m, 8H, Ar–H), 7.78 (s, 1H, –CH=), 8.71 (s, 1H, CH=N), 12.42 (s, 1H, NH), 3.78(s, 3H, OCH3); M. Formula: C18H14N2O3S; MS: m/z 338.98 (M+ + 1); Elemental analysis (CHN) Theoretical calc: C, 63.89; H, 4.17; N, 8.28 Found: C, 63.93; H, 4.17; N, 8.30 |
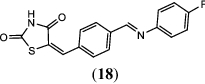 |
5-((E)-4-((E)-((4-fluorophenyl)imino)methyl)benzylidene) thiazolidine-2,4-dione: m.p. °C: 104–106; Rf value: 0.83a; % yield: 70; IR (KBr cm−1): 3431.86 (N–H str., thiazolidine ring), 1743.59 (C=O str., thiazolidin-2,4-dione ring), 1697.01 (C=N str., imine group), 1501.17 (C=C str., aromatic ring), 1612.03 (C=C str., methylene group), 3039.20 (C–H str., aromatic ring), 2925.63 (C–H str., aliphatic), 1292.50 (C–N str., thiazolidine ring), 604.82 (C–S bend., thiazolidine ring), 1152.02 (C–C str.), 1012.73 (C-F str., p-substitution on phenyl ring); 1H NMR (δ, DMSO): 6.77–7.78 (m, 8H, Ar–H), 7.77 (s, 1H, –CH=), 7.87 (s, 1H, CH=N), 12.60 (s, 1H, NH); M. Formula: C17H11N2O2SF; MS: m/z 324.85 (M+ − 1); Elemental analysis (CHN) Theoretical calc: C, 62.57; H, 3.40; N, 8.58 Found: C, 62.58; H, 3.40; N, 8.59 |
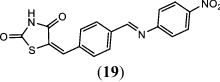 |
5-((E)-4-((E)-((4-nitrophenyl)imino)methyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 118–120; Rf value: 0.83a; % yield: 75; IR (KBr cm−1): 3369.56 (N–H str., thiazolidine ring), 1743.23 (C=O str., thiazolidin-2,4-dione ring), 1698.03 (C=N str., imine group), 1503.33 (C=C str., aromatic ring), 1601.16 (C=C str., methylene group), 3052.03 (C–H str., aromatic ring), 2925.51 (C–H str., aliphatic), 1310.97 (C–N str., thiazolidine ring), 608.61 (C–S bend., thiazolidine ring), 1163.00 (C–C str.), 1212.91 (N–O str., p-substitution on phenyl ring), 1412.67 (N=O str., p-substitution on phenyl ring); 1H NMR (δ, DMSO): 6.60–8.28 (m, 8H, Ar–H), 7.78 (s, 1H, –CH=), 8.72 (s, 1H, CH=N), 12.62 (s, 1H, NH); M. Formula: C17H11N3O4S; Elemental analysis (CHN) Theoretical calc: C, 57.79; H, 3.14; N, 11.89 Found: C, 57.80; H, 3.14; N, 11.88 |
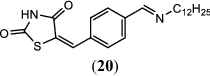 |
5-((E)-4-((E)-(dodecylimino)methyl)benzylidene)thiazolidine-2,4-dione: m.p. °C: 106–108; Rf value: 0.43a; % yield: 60; IR (KBr cm−1): 3339.52 (N–H str., thiazolidine ring), 1699.02 (C=O str., thiazolidin-2,4-dione ring), 1608.43 (C=N str., imine group), 1461.90 (C=C str., aromatic ring), 1562.34 (C=C str., methylene group), 3047.23 (C–H str., aromatic ring), 2923.03 (C–H str., aliphatic), 1296.98 (C–N str., thiazolidine ring), 608.00 (C–S bend., thiazolidine ring), 1165.06 (C–C str.); 1H NMR (δ, DMSO): 7.28–7.98 (m, 4H, Ar–H), 7.78 (s, 1H, –CH=), 8.35 (s, 1H, CH=N), 12.62 (s, 1H, NH), 1.23–1.69 (m, 20H, CH2), 0.84 (t, J = 9.00 Hz, 3H, CH3), 3.66 (m, 2H, CH2 adjacent to CH=N); M. Formula: C23H32N2O2S; MS: m/z 401.12 (M+ + 1), 399.09 (M+ − 1); Elemental analysis (CHN) Theoretical calc: C, 68.96; H, 8.05; N, 6.99 Found: C, 68.97; H, 8.05; N, 6.98 |
TLC mobile phase = a Chloroform:Methanol: 9:1
bChloroform:Toluene:GAA: 1:1:0.1
cEthyl Acetate
dMethanol:Toluene:GAA: 1:1:0.1
