Fig. 4.
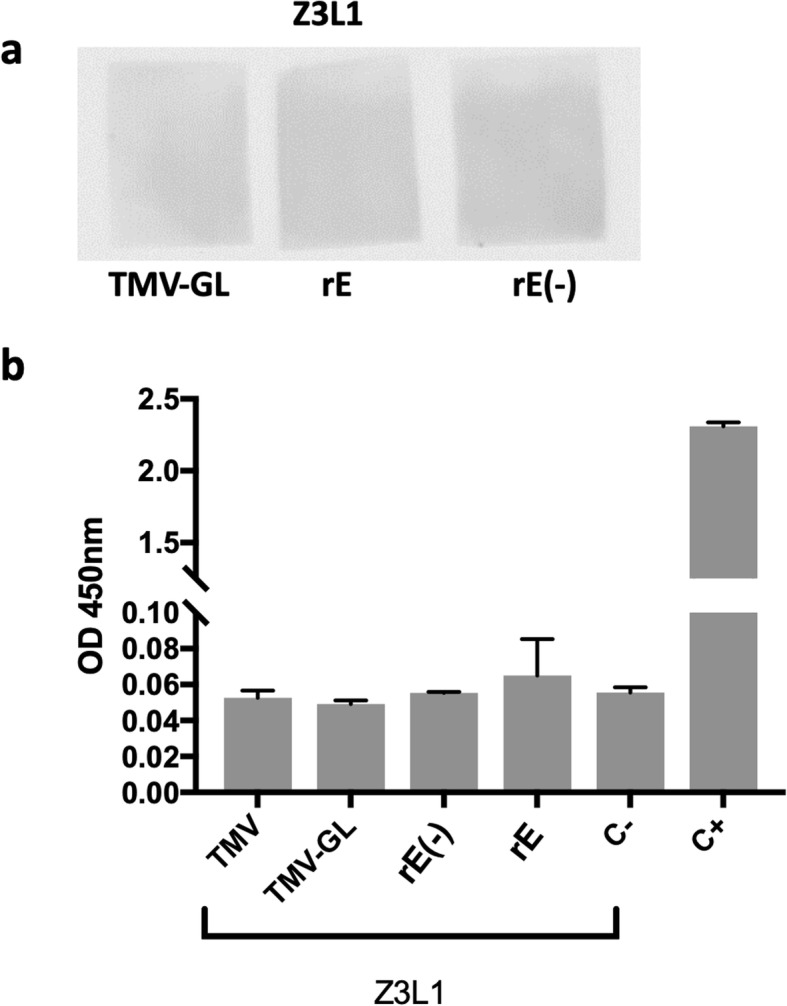
Binding characterization of Z3L1. a Binding characterization of Z3L1 by dot blot. TMV-GL, plant-made ZIKV rE, or E. coli-made ZIKV rE were dotted onto nitrocellulose, blocked, and then incubated with the human anti-ZIKV mAb Z3L1. A peroxidase-conjugated anti-human IgG secondary antibody and a chemiluminescent substrate were then used to detect any Z3L1 binding. b Binding characterization of Z3L1 by ELISA. TMV, TMV-GL, plant-made ZIKV rE (rE) and E. coli-made ZIKV rE (rE(−)) were used to coat a 96-well plate. The Z3L1 mAb or an anti-ZIKV rE serum (C+) was added, followed by incubation with HRP-conjugated secondary antibodies and the addition of OPD substrate. The data depicted are the mean absorbance at 450 nm ± SD of triplicate wells. The labels on the x-axis indicate the antigen that was plated. C- indicates that the blocking buffer was used to coat the wells instead of an antigen, thus measuring the background noise from the Z3L1 antibody binding directly to the blocked plate. C+ indicates the use of an anti-ZIKV rE antibody as a positive control with plated E. coli-made rE. (−) indicates the lack of glycosylation
