Fig. 2.
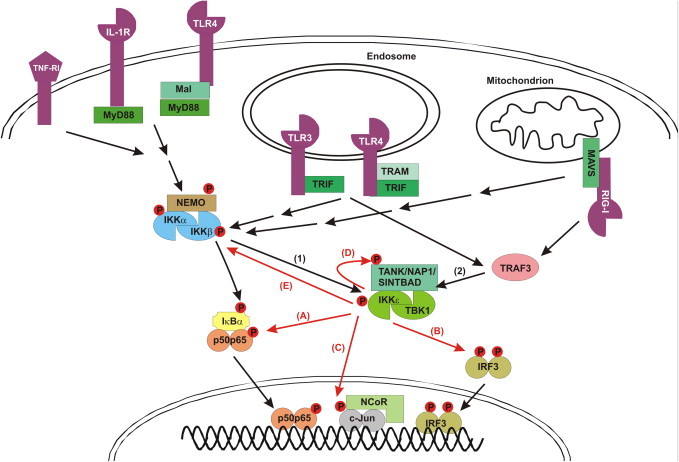
IKKɛ mediated signaling to NF-κB and IRF3 in response to specific receptors.
Non-canonical IKKs (IKKɛ and TBK1) can be activated by two signaling pathways: (1) IKKα/β mediated activation of IKKɛ/TBK1. Ligand binding to several receptors (TNF-R1, IL-1R, TLR4, RIG-I) initiates the recruitment of specific adaptor proteins (e.g. Mal, MyD88, TRAM, TRIF, MAVS), E3 ubiquitin ligases and kinases (not shown) to the receptor, eventually resulting in the activation of the canonical IKK (IKKα/IKKβ/NEMO) complex. This leads to the IKKβ-mediated phosphorylation and subsequent Lys48-linked polyubiquitination of IκBα, resulting in its proteasomal degradation and release of the p50–p65 NF-κB heterodimer, which then translocates to the nucleus. (2) IKKα/IKKβ-independent and TRAF3-dependent IKKɛ/TBK1 autoactivation. TRIF-dependent TLR3 and TLR4 signaling, as well as MAVS-dependent RIG-I signaling, induce IKKɛ/TBK1 autoactivation via TRAF3. This also requires the binding of IKKɛ/TBK1 to different scaffold proteins (TANK, NAP1, SINTBAD). IKKɛ/TBK1 then mediate different activities: (A) IKKɛ/TBK1 can phosphorylate NF-κB (p65), contributing to NF-κB dependent expression of specific genes. (B) IKKɛ/TBK1 can phosphorylate IRF3 (and IRF7; not shown), leading to its homodimerization and nuclear translocation. (C) IKKɛ/TBK1 can phosphorylate c-jun, leading to the release of the nuclear repressor complex (NCoR). (D) IKKɛ/TBK1 can phosphorylate their own scaffold proteins (TANK, NAP1 or SINTBAD), the function of which is still unclear. (E) IKKɛ/TBK1 is also able to phosphorylate the canonical IKKs, leading to their inactivation.
