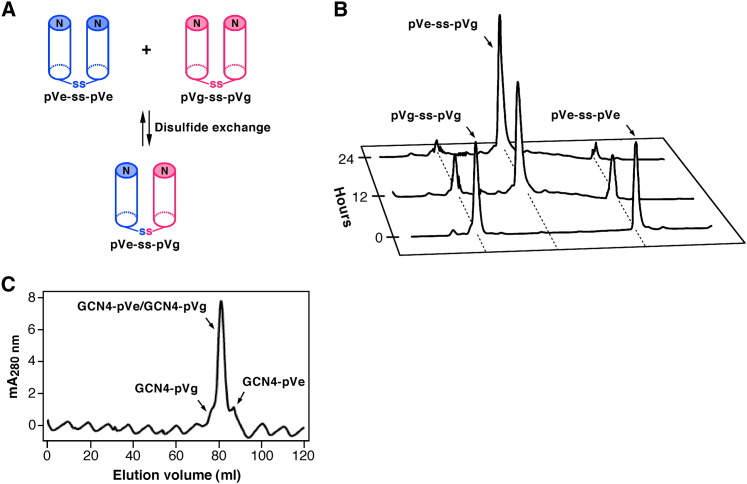
Figure 3.
Specificity of the Heterotypic Interaction between the GCN4-pVe and GCN4-pVg Peptides
(A) Preferential formation of a heterodisulfide bond. Assuming that the glycyl linkers allow for random sorting of the C-terminal cysteine residues in a mixture of the pVe-SH and pVg-SH peptides (the variants of GCN4-pVe and GCN4-pVg that have a C-terminal Gly-Gly-Cys sequence), the thermodynamically preferred heterotetramer conformation should favor oxidative heterodisulfide formation.
(B) HPLC analyses of disulfide rearrangement during the course of the equilibration under redox conditions. Disulfide exchange reactions were initiated from the disulfide-bonded pVe-ss-pVe and pVg-ss-pVg homodimers.
(C) Size-exclusion chromatography profile of the refolded GCN4-pVe/GCN4-pVg sample. An equilmolar mixture of the two peptides was refolded by renaturation from GuHCl and was analyzed by size exclusion on a Superdex 75 column equilibrated with TBS at 4°C. Fractions were analyzed by reverse-phase HPLC. Relative concentrations of the GCN4-pVe/GCN4-pVg complex and the combined GCN4-pVg and GCN4-pVe homotetramers is ∼50:1, as calculated from the peak absorbance at 280 nm.