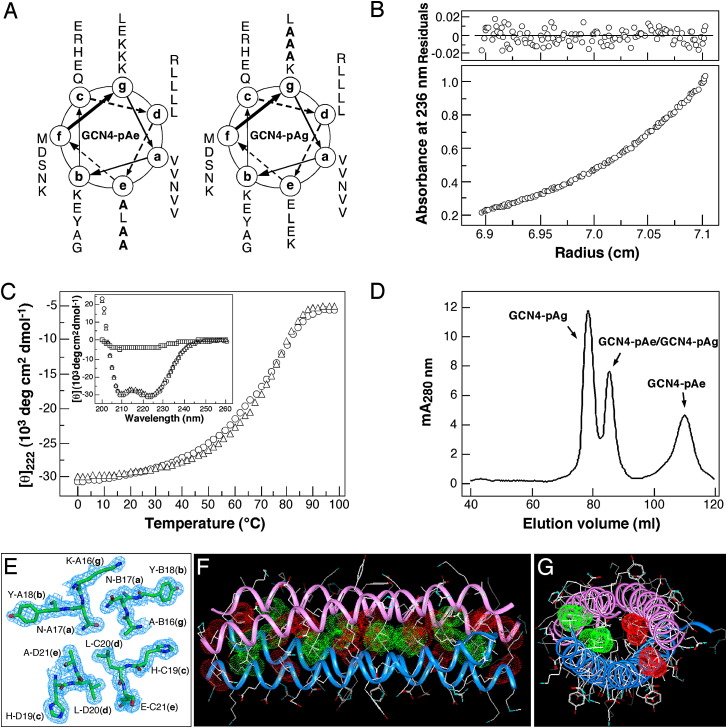
Figure 6.
Heterotetramer Formation by the GCN4-pAe and GCN4-pAg Peptides
(A) Coiled-coil helical wheel representation of the GCN4-pAe and GCN4-pAg sequences. They differ from the dimeric GCN4 leucine zipper by alanine substitutions (bold) at three e and three g positions, respectively. The sequence of GCN4-pAe is MK VKQLADK VEELLSK NYHLANE VARLAKL VGER; the sequence of GCN4-pAg is MK VKQLEDA VEELLSA NYHLENA VARLKKL VGER.
(B) Equilibrium sedimentation data (27,000 rpm) of the GCN4-pAe/GCN4-pAg mixture (150 μM) at 20°C in TBS. The data fit closely to a tetrameric complex. The deviation in the data from the linear fit for a tetrameric model is plotted.
(C) Thermal melts of the GCN4-pAe/GCN4-pAg mixture (circles) and GCN4-pAg (triangles) monitored by the CD signal at 222 nm at a total protein concentration of 10 μM. The insert shows the CD spectra of the GCN4-pAe/GCN4-pAg mixture (circles), GCN4-pAg (triangles), and GCN4-pAe (squares) at 4°C.
(D) Size-exclusion chromatography profile of the refolded GCN4-pAe/GCN4-pAg sample. An equilmolar mixture of the two peptides was refolded by renaturation from GuHCl solution and was analyzed by size exclusion on a Superdex 75 column equilibrated with TBS at 4°C. Fractions were analyzed by reverse-phase HLPC and equilibrium sedimentation. Relative concentrations of the GCN4-pVe/GCN4-pVg complex and the GCN4-pVg tetramer were calculated from the peak absorbance at 280 nm.
(E) Crystal structure of the GCN4-pAe/GCN4-pAg complex. The 1.70 Å 2Fo − Fc electron density map at 1.5σ contour shows a cross-section of the antiparallel heterotetramer.
(F) Lateral view of the antiparallel heterotetramer. The Cα backbones of GCN4-pAe (magenta) and GCN4-pAg (blue) are depicted. Red van der Waals surfaces identify residues at the a positions, and green van der Waals surfaces identify residues at the d positions.
(G) Axial view of the antiparallel heterotetramer. The view is from the N termini of helices A and B, looking down the superhelical axis. The van der Waals surfaces are colored red for Val10(a) of helices A and B and green for Leu27(d) of helices C and D.