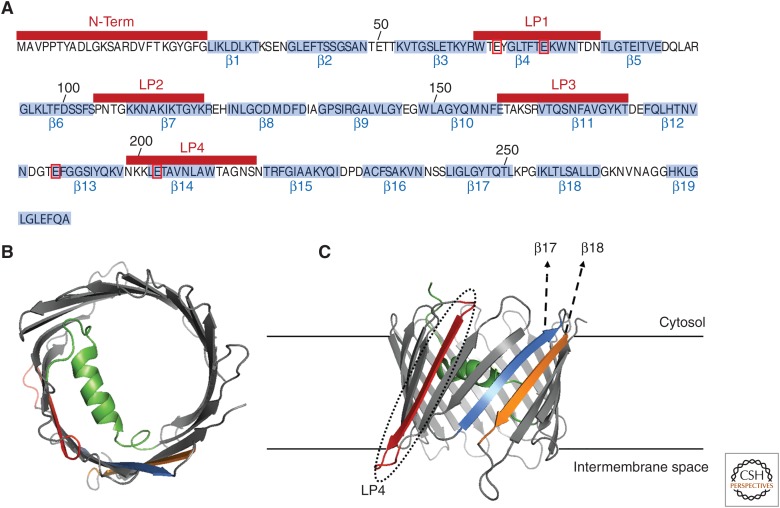
Figure 4.
Voltage-dependent anion channel 1 (VDAC1) structure. (A) Linear sequence of hVDAC1 (UniProtKB, P21796). The amino acids encompassing for the 19 b-stands (b1–b19) are highlighted in light blue. The amino-terminal (N-term) and the loop peptide (LP) regions (LP1–4) are shown above in red bars. The four key residues allegedly encompassing the Ca2+-binding properties of VDAC1 (E66, E73, E189, and E203) are boxed in red. (B,C) Color-coded views of the structure model of hVDAC1 (PDB:2K4T) with the key regions involved in its interaction with Bcl-Xl highlighted in color: N-term (green), LP4 (red), β17 (light blue), and β18 (orange). A top view is presented in B and a side view in C. The latter structure is membrane-embedded to facilitate the visualization of the possible interaction interfaces.