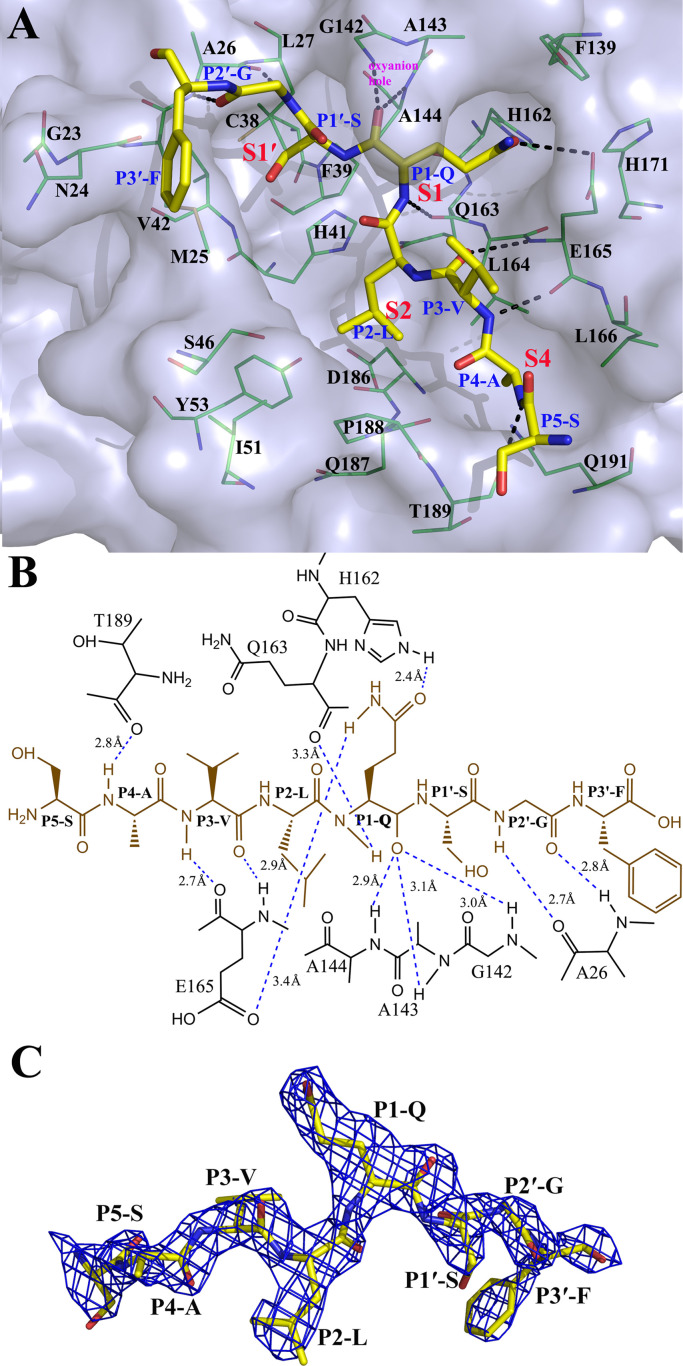
Fig. 5.
Structure of a PEDV 3CLpro variant (C144A) bound to a peptide substrate. A. Three-dimensional structure of the substrate-binding pockets with a peptide. The residues of the pockets are represented in green as a stick diagram, and the substrate is shown in yellow as a stick diagram. The S1, S2, S4 and S1′ pockets are labeled. The residues are all labeled. The nitrogen atoms are shown in blue, and oxygen atoms are shown in red. Hydrogen bond interactions are shown as black dashed lines. B. Diagram of the detailed molecular interactions between the substrate and the protease. The peptide substrate is shown in brown. Hydrogen bonds are shown as blue dashed lines, and the hydrogens were connected to their acceptors by the dashed lines. The distances labeled reflect the distances between the donor and the acceptor for all hydrogen bonds. Ceratina electron density map of the peptide substrate (2Fo-Fc, contoured at 1.0 σ).