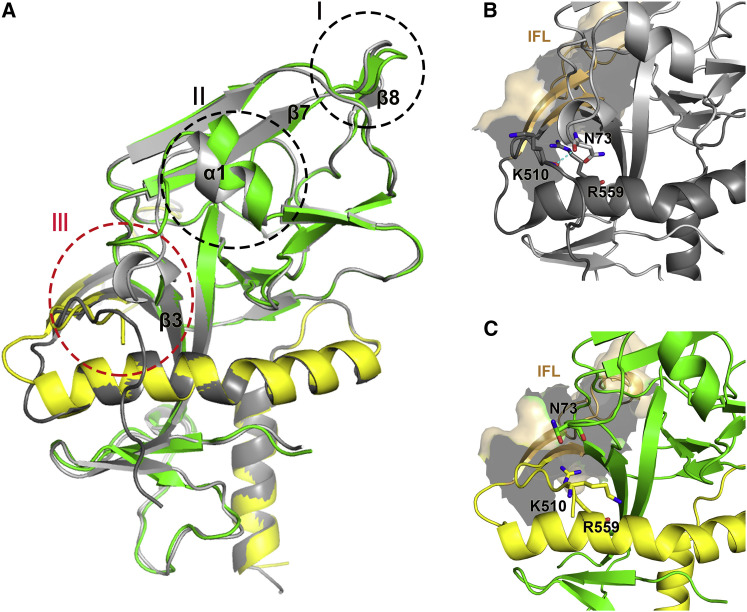
Figure 5.
Conformational Changes in the GPcl upon Enzymatic Cleavage and NPC1-C Engagement
(A) Comparison of bound GPcl (GP1 in green and GP2 in yellow) and unbound GP-ectoΔmucin (gray glycan cap is not shown here). Three conformational changes are observed in the bound GPcl, including sites I, II, and III. At site I, the β7-β8 loop moves downward; at site II, the α1 helix raises upward. Accompanied with the raising α1 helix, an upward movement of the 310 helix in the β3-α1 loop occurs at site III.
(B) Zoomed view of the site III in the unbound GP-ectoΔmucin. The residue N73 in the 310 helix could form a hydrogen bond with the residue K510 in the N-terminal portion of the IFL. This interaction could separate the electric repulsion between K510 and R559 (in the HR1 helix of GP2).
(C) Zoomed view of the site III in the bound GPcl. The upward-moved residue N73 does not form a hydrogen bond with the residue K510, and the electric repulsion between the K510 and R539 may help to release the IFL and trigger the membrane fusion.