Abstract
From an aqueous decoction of the traditional Chinese medicine “ban lan gen” (the Isatis indigotica root), an antiviral natural product CI - 39 was isolated as an NNRTI (non-nucleoside reverse transcriptase inhibitor) (EC50 = 3.40 μM). Its novel structure was determined as methyl (1-methoxy-1H-indol-3-yl)acetamidobenzoate by spectroscopic data and confirmed by single crystal X-ray diffraction. Through synthesis and structure-activity relationship (SAR) investigation of CI - 39 and 57 new derivatives (24 with EC50 values of 0.06–8.55 μM), two optimized derivatives 10f and 10i (EC50: 0.06 μM and 0.06 μM) having activity comparable to that of NVP (EC50 = 0.03 μM) were obtained. Further evaluation verified that 10f and 10i were RT DNA polymerase inhibitors and exhibited better activities and drug resistance folds compared to NVP against seven NNRTI-resistant strains carrying different mutations. Especially, 10i (EC50 = 0.43 μM) was more active to the L100I/K103N double-mutant strain as compared to both NVP (EC50 = 0.76 μM) and EFV (EC50 = 1.08 μM). The molecular docking demonstrated a possible binding pattern between 10i and RT and revealed activity mechanism of 10i against the NNRTI-resistant strains.
Keywords: Isatis indigotica, N-Alkyloxy indole derivative, HIV-1 inhibitor, Derivative synthesis, Structure-activity relationship, NNRTI
Graphical abstract
Highlights
-
•
A new antiviral natural product CI - 39 was characterized as an NNRTI from the Isatis indigotica root.
-
•
The natural product and 57 derivatives were synthesized. Of which 24 compounds showed inhibitory activity against wt HIV-1 (IC50: 0.06–8.55 μM).
-
•
Compounds 10f and 10i inhibited seven NNRTI-resistant HIV-1 strains.
-
•
Compound 10i against the HIV-1RT-L100I,K103N mutant strains was superior to EFV.
1. Introduction
Acquired immunodeficiency syndrome (AIDS) is caused by the human immunodeficiency virus (HIV) infection, which destroys the body’s immune system by infecting human immune cells and causing patients to eventually die from complications such as severe infections or secondary tumors [1,2]. In 2018, there were approximately 37.9 million people worldwide living with HIV/AIDS, including 1.7 million children [3]. Moreover, 1.7 million individuals became newly infected in 2018. As there is no AIDS vaccine available, highly active antiretroviral therapy (HAART) is still the main clinical treatment of HIV/AIDS. Many HAART comprises two or three antiretroviral drugs acting on different targets, such as reverse transcriptase (RT), protease (PR), and integrase (IN) [[4], [5], [6]]. However, long-term widespread use of HAART can lead to drug-resistant viruses and treatment failure [7]. At least 50% of patients have drug-resistant viruses. The non-nucleoside reverse transcriptase inhibitors (NNRTIs) nevirapine (NVP) and efavirenz (EFV) have been used for more than 10 years in clinical practice, and stable drug-resistant strains have emerged [8]. Clinical studies have found that after 48 weeks of etravirine and rilpivirine administration, the body produces a virus resistant to both the drugs. Several new forms of NNRTIs have been reported in recent years [[9], [10], [11], [12], [13], [14], [15], [16]]. In consideration of drug resistance continues to increase, the development of new NNRTIs is still worth the challenge.
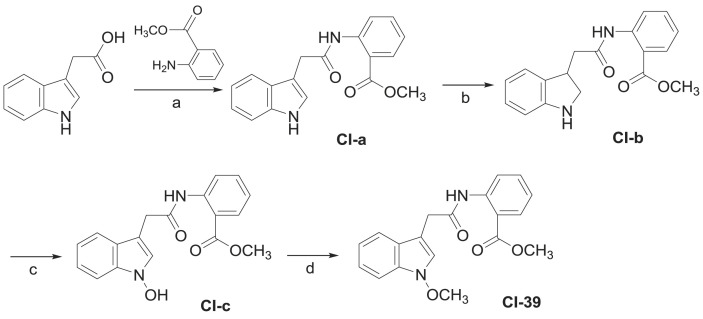
“Ban lan gen” (Isatis indigotica root) is one of the most important traditional Chinese medicines used for the treatment of influenza and various infectious diseases [17] as well as anti-severe acute respiratory syndrome (SARS) [18]. Previous studies demonstrated that indirubin, one of the bioactive indole alkaloids of the herbal drug, had the most significant cytotoxicity on IL-60 cells and inhibitory effect on pesudorabies virus (PrV) replication, while extracts from roots and leaves of I. indigotica also showed cytopathic effect (CPE) reduction either before or after infection of PrV on porcine kidney (PK-15) cells [18]. Moreover, from the ethanol extract of “da qing ye” (I. indigotica leaves) an isatisine A-derived artificial acetonide was reported to have a moderate anti-HIV-1 activity (EC50 = 37.8 μM) [19]. However as far as we know, was neither extract or purified compound from “ban lan gen” reported to inhibit HIV virus replication. Because the “ban lan gen” decoction is practically used in clinic, whereas the ethanolic extracts were mainly focused in the previous studies, we systematically studied the chemical constituents and biological activities of an aqueous extract of the I. indigotica root, resulted in characterization of more than 100 chemical constituents including around 50 alkaloids and some with antiviral (influenza virus A/Hanfang/359/95, herpes simplex virus 1, and/or Coxsackie virus B3) and cell-damage protective activities [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. A continuation of the work led to characterization of a novel anti-HIV compound methyl (1-methoxy-1H-indol-3-yl)acetamidobenzoate (CI - 39) (Fig. 1 A) with an EC50 value of 3.40 μM from a remaining subfraction. CI - 39 represents the first anti-HIV-1 indolylacetamidobenzoate derivative from I. indigotica, though from other plants a variety of natural products and extracts with inhibition of HIV-1 replication were reported [[35], [36], [37], [38], [39], [40]].
Fig. 1.
(A) Structure of the lead compound CI - 39; (B) main 1H–1H COSY (thick lines) and HMBC correlations (red arrows, from 1H to 13C); (C) crystal structure of CI - 39. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In view of the structure of CI - 39 and its inhibitory effect on the HIV-1 virus, along with the recent reports on indole derivatives with anti-HIV activity [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]], we determined that the optimization of activity and physicochemical properties by synthesis to obtain optimized lead compounds or drug candidates was worthy of further study. Because of limitation of the sample amount from the plant extract, CI - 39 was firstly synthesized with confirmation of the anti-HIV-1 replication. For the structural optimization, the effect of the following structural changes on the anti-HIV-1 activity were investigated in this study: (i) the shift of methoxyformyl on the phenylamine moiety with replacement or introduction of other functional groups; (ii) the replacement of the phenyl ring with an aromatic heterocyclic ring; (iii) the substitution of the N-methoxy group on the indole nucleus by other N-alkoxy groups; (iv) the extension or shortening of the acetamide linker between the indole and phenyl ring units. The synthetic lead compound CI - 39 and most potent derivatives were further tested for their activity against the HIV-1 strains carrying some of the most clinically relevant mutations that confer resistance to NNRTIs. Herein, reported are isolation, structural determination, and synthesis of the lead compound CI - 39, along with synthesis, anti-HIV-1 activity, and structure-activity relationship of 57 new derivatives as well as activity of three optimized compounds 6d, 10f, and 10i as novel NNRTIs against seven NNRTI-resistant HIV-1 strains carrying different RT-mutations. Molecular docking of the lead compound CI - 39 and the most potent compound 10i is also discussed.
2. Results and discussion
2.1. Chemistry
2.1.1. Isolation, structure elucidation, and antiviral assay of CI - 39
The aqueous decoction of the air-dried and pulvarized I. indigotica root was concentrated under reduced pressure and separated by column chromatography (CC) over macroporous adsorbent resin (HPD-110), eluting successively with H2O, 50% EtOH, and 95% EtOH, to yield three corresponding fractions A, B and C. Fraction C was subjected to CC over silica gel, with elution using a gradient of increasing acetone concentration (0–100%) in petroleum ether, to afford fractions C1–C11 [20]. Fraction C9 was separated successively by CC over Sephadex LH-20, reversed phase silica gel (C18), and silica gel to give a mixture, from which CI - 39 was isolated by HPLC using a semipreparative C18 column (see Experimental section 4.1.1).
Compound CI - 39 was obtained as colorless prisms in acetone. Its IR spectrum showed the presence of amino (3247 cm−1), ester and amide carbonyl (1701 and 1677 cm−1), and aromatic ring (1585 and 1511 cm−1) functionalities in the molecule. Combinatory analysis of HRMS(ESI+) and NMR spectroscopic data led to determination of the molecular formula as C19H18N2O4. In the molecular, the occurrence of a 3-substituted indole nucleus, an ortho-substituted benzene ring, two methoxy groups, an isolated methylene, and two ester and amide carbonyl carbons was deduced from analysis of the NMR spectroscopic data (Table 1 ). The connection of these structural units was further established by the 2D NMR experimental data as illustrated in Fig. 1B. Briefly a linkage between the indole nucleus and one carbonyl carbon via the methylene unit to form a 2-(1H-indol-3-yl)acetyl moiety was unambiguously established by cross-peaks H-4/H-5/H-6/H-7 in the 1H–1H COSY spectrum as well as the two- and three-bond heteronuclear correlations from H-2 to C-3, C-3a, C-7a, and C-8 and from H2-8 to C-2, C-3, C-3a, and C-9 in the HMBC spectrum, along with the chemical shifts of these proton and carbon signals (Fig. 1B). Location of an methoxyformyl at the ortho-substituted benzene ring to construct an methyl ortho-substituted benzoate moiety was deduced from the 1H–1H COSY coupling correlations of H-3′/H-4′/H-5′/H-6′ and the long-range HMBC correlations from H-3′ to C-1′ and C-5′ and from H-6′ to C-2′, C-4, and C-7′ as well as from OCH 3 to C-7′. To satisfy the requirement of the molecular composition, the remaining methoxy unit must be located at the N atom of the 2-(1H-indol-3-yl)acetyl moiety, in turn which must link via an amide bond to the only opened position of the methyl ortho-substituted benzoate moiety. The deduction was finally proved by X-ray diffraction analysis of a suitable single crystal crystallized in the acetone solution of CI - 39, an ORTEP drawn of the crystal structure shown in Fig. 1C. Thus, the structure of CI - 39 was determined as methyl (1-methoxy-1H-indol-3-yl)acetamidobenzoate.
Table 1.
The NMR spectroscopic data (δ) for CI - 39 in Me2CO-d6a.
| no. | δH | δC | no. | δH | δC |
|---|---|---|---|---|---|
| 1 | 1′ | 116.1 | |||
| 2 | 7.63 s | 124.3 | 2′ | 142.3 | |
| 3 | 105.1 | 3′ | 8.74 d (8.5) | 120.6 | |
| 3a | 124.8 | 4′ | 7.55 dd (9.0, 8.5) | 135.1 | |
| 4 | 7.59 d (8.0) | 119.8 | 5′ | 7.09 dd (9.0, 8.0) | 123.1 |
| 5 | 7.06 dd (8.0, 7.5) | 120.7 | 6′ | 7.91 dd (8.0) | 131.5 |
| 6 | 7.22 dd (7.5, 8.0) | 123.3 | 7′ | 168.7 | |
| 7 | 7.46 d (8.0) | 109.0 | NH | 10.92 s | |
| 7a | 133.4 | OCH3 | 3.72 s | 52.6 | |
| 8 | 3.87 s | 35.7 | NOCH3 | 4.19 s | 66.4 |
| 9 | 170.7 |
Chemical shift values (δ) were measured at 500 MHz for 1H and at 125 MHz for13C, respectively. Proton coupling constants (J) in Hz are given in parentheses. The assignments were based on DEPT, 1H–1H COSY, HSQC, and HMBC experiments.
According to clinic application of “Ban lan gen” (I. indigotica root) in traditional Chinese medicine [17], anti-viral activity of CI - 39 was assayed using cell-based protocols as reported in our previous publications [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34],56]. This compound showed significant effects on H3N2 (influenza virus A/Hanfang/359/95, EC50 = 2.59 μM), HSV-1 (herpes simplex virus 1, EC50 = 0.95 μM), Coxsackie virus B3 (EC50 = 3.70 μM), and HIV-1 (EC50 = 3.40 μM).
2.1.2. Synthesis ofCI-39and its inhibitory activity against RT RNA-dependent DNA polymerase
The synthesis of CI - 39 is shown in Scheme 1 . Methyl 2-(2-(1H-indol-3-yl)acetamido)benzoate (CI-a) was obtained through an amidation reaction of indole-3-carboxylic acid with 2-aminobenzoate. Methyl 2-(2-(indolin-3-yl)acetamido)benzoate (CI-b) was prepared through reduction using trifluoroacetic acid and triethylsilane [57]. The oxidation of CI-b was carried out by sodium tungstate and hydrogen peroxide to produce methyl 2-(2-(1-hydroxy-1H-indol-3-yl)acetamido)benzoate (CI-c) [58]. Then, the methylation of CI-c with trimethylsilyldiazomethane produced CI - 39.
Scheme 1.
Synthesis of the natural product CI - 39. Reagents and conditions: (a) EDCI, DMAP, CH2Cl2, r.t.; (b) Et3SiH, CF3COOH, reflux; (c) sodium tungstate dihydrate, hydrogen peroxide (30%), MeOH, 0 °C then 15 °C; (d) (trimethylsilyl)diazomethane, CH2Cl2, r.t.
With the synthesized CI - 39, inhibitory activities against RT RNA-dependent DNA polymerase [59] and ribonuclease H [60] were detected, along with an activity test against VSVG/HIV-1 replication [56]. The result (Table 3) indicated that CI - 39 was an inhibitor of the RT RNA-dependent DNA polymerase with the IC50 value of 7.20 μM, but inactive to ribonuclease H (IC50 > 30 μM).
Table 3.
Effects of CI - 39, 6d, 10f, and 10i on HIV-1 RT DNA polymerase and ribonuclease H activities.
| Compd | IC50±SD, μM |
||
|---|---|---|---|
| DNA polymerase | Ribonuclease H | VSVG/HIV-1 replication | |
| CI - 39 | 7.20 ± 0.50 | >30 | 3.37 ± 0.45 |
| 6d | 0.27 ± 0.03 | >100 | 0.49 ± 0.11 |
| 10f | 0.11 ± 0.04 | >100 | 0.07 ± 0.02 |
| 10i | 0.08 ± 0.005 | >100 | 0.04 ± 0.01 |
| HQD | NT | 2.81 ± 0.15 | NT |
| NVP | 0.98 ± 0.53 | NT | 0.02 ± 0.003 |
| EFV | 0.01 ± 0.003 | NT | 0.001 ± 0.0001 |
IC50: half maximal inhibitory concentration; NT: not tested.
2.1.3. Structural modification of CI - 39
The synthesis of N-alkoxy indole derivatives is shown in Scheme 2 . The first two steps to obtain the intermediates 2, 3, 7 and 8 are as same as the method of preparing CI-a, CI-b. The oxidation of 3 was carried out by m-CPBA to produce an indole N-hydroxyl derivative (4) [61]. The methylation of 4 with trimethylsilyldiazomethane produced the target product (5), and 6 was synthesized from 4 in the presence of potassium carbonate and a halogenated alkane. The intermediates 8 were transformed into the indole N-hydroxyl derivative (9) through an oxidation reaction using sodium tungstate and hydrogen peroxide. Then, alkylation of 9 under the basic condition provided 10 (Scheme 3 ).
Scheme 2.
Synthesis of compounds 5a-5w and 6a-6q. Reagents and conditions: (a) EDCI, DMAP, CH2Cl2, r.t.; (b) Et3SiH, CF3COOH, reflux; (c) m-CPBA, MeOH, 0 °C, then r.t.; (d) trimethylsilyldiazomethane, CH2Cl2, r.t.; (e) K2CO3, DMF, r.t..
Scheme 3.
Synthesis of compounds 10a-10j. Reagents and conditions: (a) EDCI, DMAP, CH2Cl2, r.t.; (b) Et3SiH, CF3COOH, reflux; (c) sodium tungstate dihydrate, hydrogen peroxide (30%), MeOH, 0 °C then 15 °C; (d) (trimethylsilyl)diazomethane, CH2Cl2, r.t. or K2CO3, DMF, r.t..
The synthesis of 14 and 15 is depicted in Scheme 4 . The intermediate (13) was prepared sequentially by oxidation of 11 and alkylation of the resulting product 12. A reaction of 13 with oxalyl chloride and 3-chloro-4-aminopyridine yielded 14, which was further transformed into 15 by reduction with sodium borohydride. The reaction of indole (16) and pyridinecarboxaldehyde under the basic condition [62] afforded 17, which was sequentially reduced and oxidized to yield the intermediates 19 via 18. Then, propylation of 19 synthesized 20 (Scheme 5 ).
Scheme 4.
Synthesis of compounds 14 and 15. Reagents and conditions: (a) sodium tungstate dihydrate, hydrogen peroxide (30%), MeOH, 0 °C then 15 °C; (b) K2CO3, DMF, r.t.; (c) (COCl)2, Et2O, 0 °C to r.t.; then TEA, 4-amino-3-chloropyridine, CH2Cl2, 0 °C to r.t.; (d) sodium borohydride, MeOH, r.t..
Scheme 5.
Synthesis of target compounds 20a-20c. Reagents and conditions: (a) NaOH, MeOH, r.t.; (b) Et3SiH, CF3COOH, reflux; (c) sodium tungstate dihydrate, hydrogen peroxide (30%), MeOH, 0 °C then 15 °C; (d) K2CO3, DMF, r.t..
2.1.4. Structure-activity relationships
All the synthesized CI - 39 and 58 derivatives were tested against replication of the wild-type HIV-1 virus and their cytotoxicities on HEK 293T cell as well. As shown in Table 2 , replacement of either the N–OMe in CI - 39 by N–H (CI-a) and N–OH (CI-c) or the indole by an indoline (CI-b) resulted in loss of activity. This indicates that the N-substituted indole moiety is one of the key pharmacophores. Among the phenylamine ring substituted derivatives (5), only those with ortho-halogen atoms Cl, Br, and I (5j, 5m, and 5n) retained activity with the EC50 values of 3.30, 3.20, and 4.40 μM, respectively, whereas all the others (5a−5i, 5k, 5l, and 5o−5u) as well as those with the acetyl linker replaced by propionyl and butyryl (5v and 5w) resulted in loss of activity. Accordingly, ortho-substitution at the phenylamine moiety by the electron-withdrawing groups (COOMe, Cl, Br, and I) and retention of the acetyl linker are essential to maintain activity.
Table 2.
The effect of the compounds against wild type HIV-1 replicationa.
| Compd | Ar | R1 | R2 | EC50±SD, μM | CC50±SD, μM | SI |
|---|---|---|---|---|---|---|
| CI - 39 |  |
–OCH3 | – | 3.40 ± 0.45 | >30; (51.95 ± 4.29%) | >8.82 |
| CI-a | 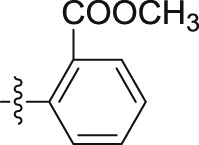 |
–H | – | >10; (91.98 ± 0.02%) | >30; (65.50 ± 5.49%) | – |
| CI-ba | – | – | – | >10; (82.96 ± 1.07%) | >30; (71.46 ± 4.41%) | – |
| CI-c | 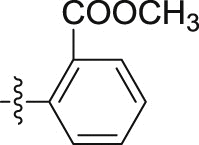 |
–OH | – | >10; (107.10 ± 7.63%) | 24.60 ± 3.79 | – |
| 5a |  |
–OCH3 | – | >10; (113.22 ± 0.48%) | 27.98 ± 5.24 | – |
| 5b |  |
–OCH3 | – | >10; (53.31 ± 3.18%) | >30; (62.74 ± 0.54%) | – |
| 5c | 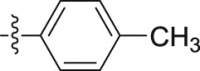 |
–OCH3 | – | >10; (124.35% ± 9.01%) | >30; (52.47 ± 5.34%) | – |
| 5d | 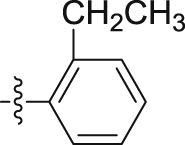 |
–OCH3 | – | >10; (64.33 ± 0.23%) | >30; (68.50 ± 3.46%) | – |
| 5e | 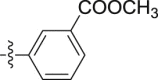 |
–OCH3 | – | >10; (109.33 ± 7.81%) | >30; (50.46 ± 1.22%) | – |
| 5f | 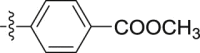 |
–OCH3 | – | >10; (104.33 ± 3.96%) | >30; (72.64 ± 4.42%) | – |
| 5g | 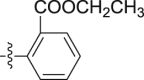 |
–OCH3 | – | >10; (60.76 ± 0.94%) | 29.08 ± 0.07 | – |
| 5h | 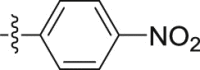 |
–OCH3 | – | >10; (81.41 ± 3.79%) | >30; (54.00 ± 3.54%) | – |
| 5i | 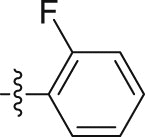 |
–OCH3 | – | >10; (72.89 ± 1.06%) | 28.58 ± 1.78 | – |
| 5j | 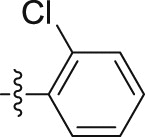 |
–OCH3 | – | 3.30 ± 0.30 | 27.78 ± 3.57 | 8.42 |
| 5k | 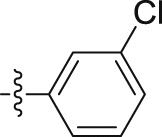 |
–OCH3 | – | >10; (113.00 ± 3.13%) | >30; (61.02 ± 5.24%) | – |
| 5l | 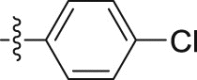 |
–OCH3 | – | >10; (103.55 ± 3.27%) | >30; (51.01 ± 2.25%) | – |
| 5m |  |
–OCH3 | – | 3.20 ± 0.40 | >30; (83.48 ± 5.27%) | >9.38 |
| 5n | 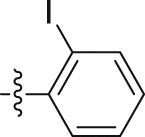 |
–OCH3 | – | 4.40 ± 0.45 | >30; (67.04 ± 0.06%) | >6.82 |
| 5o | 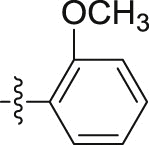 |
–OCH3 | – | >10; (71.46 ± 4.62%) | >30; (59.57 ± 0.05%) | – |
| 5p | 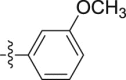 |
–OCH3 | – | >10; (104.29 ± 0.82%) | >30; (86.68 ± 6.56%) | – |
| 5q | 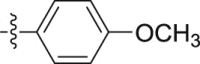 |
–OCH3 | – | >10; (128.61 ± 0.08%) | >30; (86.09 ± 7.19%) | – |
| 5r | 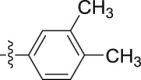 |
–OCH3 | – | >10; (95.02 ± 2.76%) | >30; (51.62 ± 0.34%) | – |
| 5s | 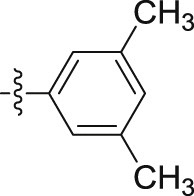 |
–OCH3 | – | >10; (87.28 ± 5.47%) | >30; (62.62 ± 4.79%) | – |
| 5t | 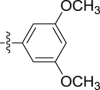 |
–OCH3 | – | >10; (98.07 ± 0.36%) | 21.63 ± 0.07 | – |
| 5u | 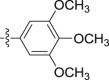 |
–OCH3 | – | >10; (47.94 ± 3.62%) | 13.86 ± 1.25 | – |
| 5vb | – | – | – | >10; (74.25 ± 7.59%) | 26.76 ± 1.50 | – |
| 5wc | – | – | – | >10; (82.07 ± 1.79%) | >30; (61.31 ± 3.32%) | – |
| 6a | 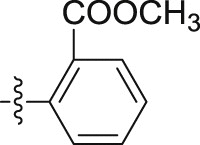 |
–OCH2CH3 | – | 5.70 ± 0.10 | >30; (52.76 ± 2.56%) | >5.26 |
| 6b | 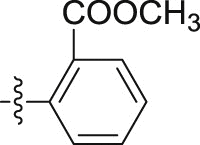 |
–OCH2CH2CH3 | – | 6.70 ± 0.50 | >30; (55.39 ± 2.79%) | >4.48 |
| 6c | 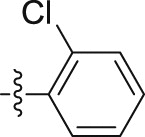 |
–OCH2CH3 | – | 0.86 ± 0.04 | 20.81 ± 2.13 | 24.20 |
| 6d | 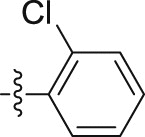 |
–OCH2CH2CH3 | – | 0.29 ± 0.01 | 29.49 ± 1.37 | 101.69 |
| 6e | 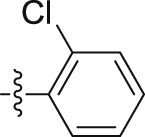 |
–O(CH2)3CH3 | – | 0.64 ± 0.03 | 29.14 ± 0.74 | 45.53 |
| 6f | 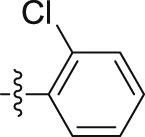 |
–O(CH2)4CH3 | – | 0.63 ± 0.03 | 18.73 ± 2.18 | 29.73 |
| 6g | 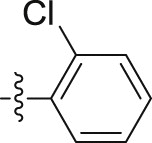 |
–O(CH2)5CH3 | – | 1.02 ± 0.08 | >30; (56.95 ± 2.10%) | >29.41 |
| 6h | 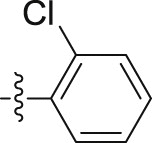 |
–O(CH2)6CH3 | – | 1.95 ± 0.05 | 23.88 ± 1.90 | 12.25 |
| 6i | 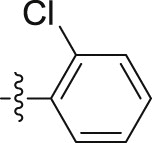 |
–O(CH2)7CH3 | – | 4.40 ± 0.50 | 22.93 ± 3.21 | 5.21 |
| 6j | 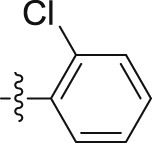 |
–O(CH2)8CH3 | – | >10; (58.30 ± 1.62%) | 28.85 ± 1.16 | – |
| 6k | 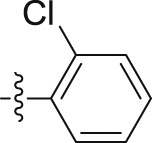 |
–O(CH2)9CH3 | – | >10; (97.73 ± 1.22%) | >30; (52.80 ± 0.76%) | – |
| 6l | 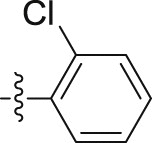 |
 |
– | 8.55 ± 0.95 | 16.50 ± 1.79 | 1.93 |
| 6m | 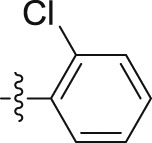 |
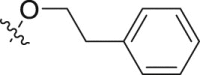 |
– | 5.50 ± 0.60 | >30; (49.31 ± 3.58%) | >5.45 |
| 6n | 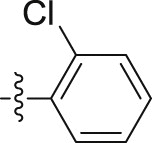 |
 |
– | >10; (73.20 ± 8.14%) | 20.28 ± 236 | – |
| 6o | 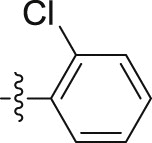 |
 |
– | >10; (146.89 ± 10.59%) | 28.06 ± 0.11 | – |
| 6p | 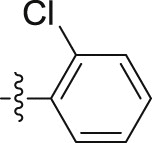 |
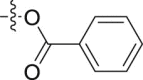 |
– | >10; (113.06 ± 0.17%) | 29.43 ± 0.15 | – |
| 6q | 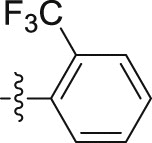 |
–OCH2CH2CH3 | – | 4.28 ± 0.41 | 24.85 ± 3.06 | 5.81 |
| 10a | 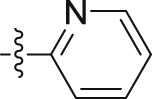 |
–OCH3 | – | >10; (138.11 ± 7.25%) | >30; (63.67 ± 4.32%) | – |
| 10b | 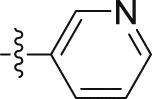 |
–OCH3 | – | >10; (118.26 ± 7.86%) | >30; (78.70 ± 0.91%) | – |
| 10c | 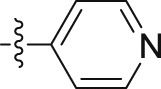 |
–OCH3 | – | 4.95 ± 0.35 | 14.27 ± 0.15 | 2.88 |
| 10d | 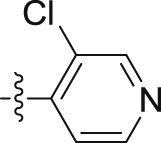 |
–OCH3 | – | 0.53 ± 0.01 | 19.96 ± 0.86 | 37.66 |
| 10e | 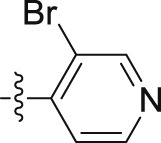 |
–OCH3 | – | 0.66 ± 0.06 | 18.55 ± 0.58 | 28.11 |
| 10f | 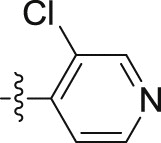 |
–OCH2CH2CH3 | – | 0.06 ± 0.0005 | 16.41 ± 1.32 | 273.50 |
| 10g | 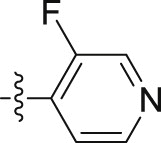 |
–OCH2CH2CH3 | – | 0.33 ± 0.02 | 14.13 ± 0.13 | 42.82 |
| 10h | 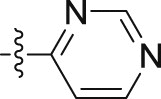 |
–OCH2CH2CH3 | – | 3.30 ± 0.00 | 17.92 ± 0.01 | 5.43 |
| 10i | 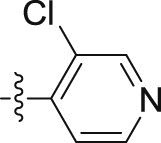 |
–O(CH2)3CH3 | – | 0.06 ± 0.0005 | 22.06 ± 2.01 | 367.67 |
| 10j | 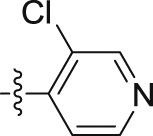 |
–O(CH2)4CH3 | – | 0.13 ± 0.00 | 21.96 ± 1.99 | 168.92 |
| 14 | – | – | 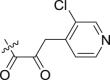 |
>10; (131.6 ± 0.60%) | 20.92 ± 5.11 | – |
| 15 | – | – | 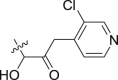 |
2.01 ± 0.06 | 18.98 ± 2.31 | 9.44 |
| 20a | – | – | 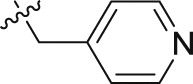 |
>10; (99.48 ± 1.49%) | >30; (69.96 ± 0.41%) | – |
| 20b | – | – | 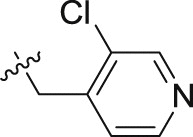 |
>10; (65.67 ± 2.95%) | >30; (67.37 ± 6.75%) | – |
| 20c | – | – | 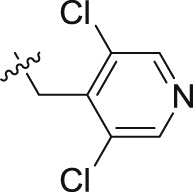 |
>10; (93.41 ± 1.56%) | >30; (77.47 ± 3.25%) | – |
| NVP | – | – | – | 0.03 ± 0.003 | >30; (93.26 ± 0.68%) | >1000.00 |
| EFV | – | – | – | 0.0007 ± 0.0002 | 23.72 ± 4.45 | 33885.71 |

a: CI-b = 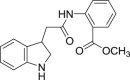 ; b: 5v =
; b: 5v = 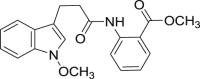 ; c: 5w =
; c: 5w = 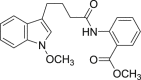 .
.
EC50: half maximal effective concentration; CC50: 50% cytotoxic concentration; SI: selectivity index.
The percentage in parentheses represents the infectivity or cell viability when treated with the compound at a final concentration of 10 μM or 30 μM.
For the N-alkoxy-substituted derivatives of CI - 39, activity was decreased by prolonging the linear alkyl chain (6a and 6b). However, for the halogenated analogues of 5j, activity was enhanced by extending of the linear alkyl chain from one to five carbon atoms, e.g. methoxy (5j: EC50 = 3.30 μM), ethoxy (6c: EC50 = 0.86 μM), propoxy (6d: EC50 = 0.29 μM), n-butoxy (6e: EC50 = 0.64 μM), and n-pentyloxy (6f: EC50 = 0.63 μM). When the number of alkyl linear carbon atoms exceeded to six, the HIV-1 virus replication inhibitions were gradually decreased (6g: EC50 = 1.02 μM; 6h: EC50 = 1.95 μM; 6i: EC50 = 4.40 μM), and completely lost when the carbon atom number reached to 9 (6j). In addition, introduction of the bulky units at the N-atom of the indole moiety also weakened activity, e.g. benzyloxy (6l: EC50 = 8.55 μM), phenethyloxy (6m: EC50 = 5.50 μM), and p-methoxybenzyloxy (6n). Complete loss of activity was observed also when electron-withdrawing acetyloxy or benzyloxy substituted at the N-atom of the indole moiety (6o and 6p). Interestingly, as compared with 6d (EC50 = 0.29 μM), the potency was decreased by ortho-substitution of the electron-withdrawing group CF3 on the phenylamine moiety (6q: EC50 = 4.28 μM), which differed from 5j, 5m, and 5n. The aforementioned results indicate that the N-alkoxy in the CI - 39 derivatives is required for activity against the HIV-1 virus replication, and the best activity is gained when the number of the linear alkyl carbon atoms is three (6d: EC50 = 0.29 μM).
With replacement of the phenylamine moiety by pyridinamines (10a-10c), activity was retained only for the derivative of pyridine-4-amine (10c: EC50 = 4.95 μM). Simultaneous introduction of Cl or Br at the ortho-position of the amino group in the pyridine-4-amine moiety led to a remarkable gain of activity by almost an order of magnitude (10d: EC50 = 0.53 μM and 10e: EC50 = 0.66 μM). Further substitution of N-methoxy by N-propoxy (10f: EC50 = 0.06 μM) or N-butoxy (10i: EC50 = 0.06 μM) increased activity by one more order of magnitude, while relatively less increase of activity was observed by substitution of N-methoxy with N-pentyloxy (10j: EC50 = 0.13 μM). In addition, as compared with 10f, activity was reduced by an ortho-fluorine substituent on the pyridine-4-amine moiety (10g: EC50 = 0.33 μM) or by hydroxylation of the acetyl linker (15: EC50 = 2.01 μM). Moreover, comparison of 6d (EC50 = 0.29 μM) and 10h (EC50 = 3.30 μM) indicated that replacement of 2-chloroaniline by pyrimidin-4-amine reduced activity by an order of magnitude. Further modification of the acetyl linker through oxidation (14) and replacement with a methylene unit (20) resulted in the disappearance of activity. Therefore, in the synthesized derivatives the 3-chloropyridin-4-amine moiety is optimal to replace the methyl 2-aminobenzoate moiety in the natural product CI - 39. The three potent compounds 6d, 10f, and 10i were selected for following investigations. Notably, we discovered that the intermediate 4o (ZT55, inactive to HIV-1) was a highly-selective tyrosine kinase inhibitor of JAK2V617F against myeloproliferative neoplasms [63] while other derivatives were inactive at 10 μM. As shown in Table 2, all compounds cytotoxicity was tested, and the cell viabilities were influenced somehow by treating with the compounds at the maximum concentration of 30 μM; nevertheless, the CC50 of all 59 compounds was higher than 10 μM, which was the highest concentration on anti-HIV replication assay.
2.2. Biology
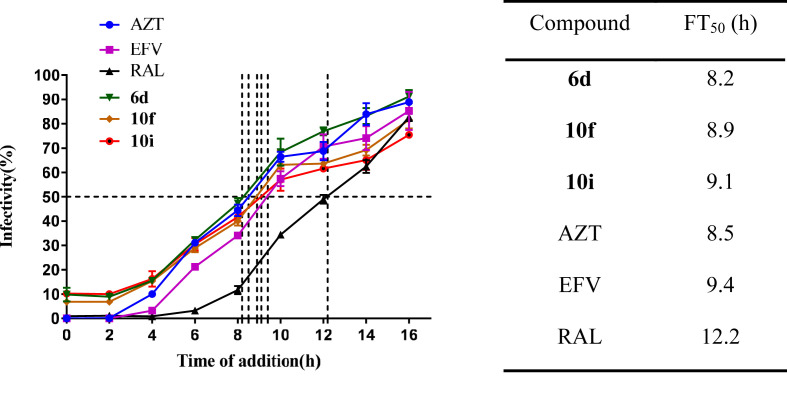
The time-of-drug addition (TOA) assay was conducted to identify the stage at which the active compounds had the inhibitory effects on HIV-1. The reverse transcriptase inhibitors zidovudine (AZT) and EFV, and the integrase inhibitor raltegravir (RAL) were used as reference drugs. The assay showed that the activity lost curves of 6d, 10f, and 10i were close to those of AZT and EFV. They had a range of time of 50% failure (FT50) from 8.2 to 9.1 h comparable to AZT at 8.5 h and EFV at 9.4 h, which were far ahead of the integrase inhibitor RAL with a FT50 of 12.2 h (Fig. 2 ), indicating that compounds 6d, 10f and 10i inhibit HIV-1 replication at the reverse transcription step.
Fig. 2.
Time-of-drug addition assay.
Compounds were added at different time points of VSVG/HIV-1 post-infection. The infected cells were lysed 48h post-infection and luciferase activity was measured by FB12 luminometer. The test compounds 6d, 10f and 10i, exhibited the time of 50% failure (FT50) at 8.2, 8.9, and 9.1 h, respectively; the FT50s of reference compounds AZT, EFV, and RAL were 8.5, 9.4, and 12.2 h, respectively.
Reverse transcription is a process of generating double-stranded proviral DNA with an HIV-1 RNA genome as the template. This complex process is preceeded by RT catalysis with DNA polymerase and Ribonuclease H activities, during which (−)DNA strand is generated by DNA polymerase activity based on the template RNA and the template RNA is hydrolyzed by Ribonuclease H [64,65]. Since the lead compound CI - 39 was proved as a RT DNA polymerase inhibitor, we also tested the effects of compounds 6d, 10f and 10i on both HIV-1 RT DNA polymerase and Ribonuclease H activities. As shown in Table 3 , all three compounds displayed inhibitory activity on RT RNA-dependent DNA polymerase, but not on Ribonuclease H, which indicated that compounds 6d, 10f, and 10i were RT DNA polymerase inhibitors.
HIV-1 RT comprises two subunits p66 and p51. The p66 is the catalytic subunit designated as fingers, palm, thumb, connection, and RNase H domains [66]. All members of approved NNRTIs are RT-DNA polymerase inhibitors by binding to a hydrophobic pocket located between the base of the thumb and the β-sheets of the palm domain, about 10 Å under the catalytic active center. Due to the low fidelity of HIV-1 replication and over two decades of NNRTIs use in clinic, the NNRTI-resistant mutants have developed. Among these, K103N, Y181C or Y188L, carried by RT, are the most prevalent mutants [67]. Lys103 locates on the outer rim of the NNRTI-binding pocket and in the vicinity of the entrance to the pocket; Tyr181 and Tyr188 locates at NNRTI-binding pocket. When Lys103 changes to asparagine, the RT catalytic pocket is preferred in its closed form, resulting in less electrostatic forces within the NNRTIs [68]. Both Tyr181 and Tyr188 exist at the catalytic pocket, which the NNRTIs act on through the π-π interaction of their aromatic rings. As the mutations of Y181C or Y188L occur, the forces between NNRTIs and RT by π-π interaction disappear [69]. For novel NNRTIs, the activity against existing NNRTI-resistant HIV-1 is a pivotal characteristic.
In this study, we evaluated the activities of the natural product CI - 39 and the potent derivatives 6d, 10f, and 10i against replication of the seven NNRTI-resistant HIV-1 strains. Overall, 10f and 10i exhibited better activities and drug resistance folds compared to NVP against six of the seven resistant strains (Table 4 ). Particularly, 10i (EC50 = 0.43 μM) was more notably more active against the double mutant L100I/K103N comparing to both NVP (EC50 = 0.76 μM) and EFV (EC50 = 1.08 μM). It is worth noting that the natural product CI - 39 is the only one fully active on all the viral mutants though it did not exhibit the best activity among the four tested compounds.
Table 4.
Effects of CI - 39, 6d, 10f, and 10i on wild type and NNRTI-resistant HIV-1 replicationa.
| Virus | EC50±SD, μM |
|||||
|---|---|---|---|---|---|---|
| CI-39 | 6d | 10f | 10i | NVP | EFV | |
| VSVG/HIV-1wt | 3.37 ± 0.45 (1.0) | 0.49 ± 0.11(1.0) | 0.07 ± 0.02 (1.0) | 0.04 ± 0.01 (1.0) | 0.02 ± 0.003 (1.0) | 0.001 ± 0.0001 (1.0) |
| VSVG/HIV-1RT-K103N | 3.00 ± 0.00 (0.9) | >10 (>20.4) | 0.70 ± 0.22 (9.7) | 0.72 ± 0.03 (18.7) | 1.40 ± 0.19 (81.2) | 0.03 ± 0.003 (27.0) |
| VSVG/HIV-1RT-Y181C | 2.41 ± 0.02 (0.7) | 2.13 ± 0.42 (4.3) | 0.18 ± 0.03 (2.5) | 0.04 ± 0.01 (1.0) | 2.58 ± 0.31 (149.9) | 0.002 ± 0.0001 (1.7) |
| VSVG/HIV-1RT-K103N,Y181C | 3.08 ± 0.66 (0.9) | >10 (>20.4) | 1.85 ± 0.33 (25.7) | 0.69 ± 0.08 (17.7) | 80.00 ± 9.89 (4657.9) | 0.05 ± 0.003 (50.2) |
| VSVG/HIV-1RT-L100I,K103N | 2.14 ± 0.29 (0.6) | 8.95 ± 1.21 (18.2) | 1.18 ± 0.11 (16.3) | 0.43 ± 0.04 (11.0) | 0.76 ± 0.06 (44.4) | 1.08 ± 0.19 (1080.3) |
| VSVG/HIV-1RT-Y188L | 3.27 ± 0.44 (1.0) | >10 (>20.4) | 1.89 ± 0.22 (26.1) | 1.42 ± 0.03 (36.7) | >100 (>5000.0) | 0.12 ± 0.03 (124.3) |
| VSVG/HIV-1RT-K103N,G190A | 3.19 ± 0.40 (0.9) | >10 (>20.4) | 1.38 ± 0.25 (19.2) | 0.70 ± 0.11 (18.1) | 14.57 ± 0.91 (848.0) | 0.18 ± 0.01 (183.0) |
| VSVG/HIV-1RT-K103N,V108I | 4.07 ± 0.08 (1.2) | >10 (>20.4) | 1.27 ± 0.10 (17.6) | 0.56 ± 0.01 (14.5) | 2.87 ± 0.35 (167.0) | 0.06 ± 0.01 (55.5) |
Mean change (fold) in EC50 compared to wild type.
2.3. Molecular modeling
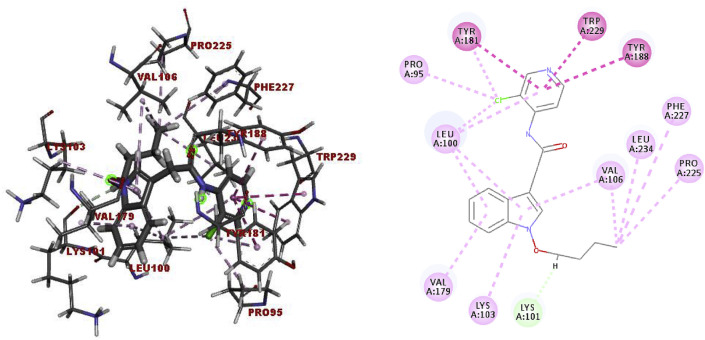
In order to better understand the possible binding mode of the active compounds, the potent 10i and CI - 39 as well as the positive controls NVP and EFV were docked into the HIV-1 WT RT (PDB code: 1TL1) [69] and the HIV-1 RT mutants RT-Y181C (PDB code: 1JLB) [70], RT-K103N/Y181C (PDB code: 5VQY) [71], and RT-L100I/K103N (PDB code: 2ZE2) [72], respectively. The docking results of 10i into WT RT (Fig. 3 ) showed: (i) π-alkyl interactions between the indole ring with Lys103, Leu100, Val179, and Val106; (ii) alkyl interactions between the end of the butoxy chain with Val106, Leu234, Phe227, and Pro225, respectively, in addition to an H-bond between the oxygen-germinal hydrogen on the N-alkoxy chain and the Lys101 carbonyl oxygen; (iii) π-π interactions between the pyridine ring with Tyr181, Tyr188, and Trp229, respectively; and (iv) interactions between chlorine with the Tyr181, Pro95, and Leu100 residues. The docking into the Y181C mutated RT (1JLB) (Fig. 4 a) indicated that the interaction between chlorine and Tyr181 in WT RT was replaced by a π-alkyl interaction between the pyridine ring and Cys181, while Tyr188 retained the π-π interaction with the pyridine ring. In addition, the pyridine ring lost its acting force with Leu100, in turn which had a π-sigma interaction with the indole ring. Differently the docking result of CI - 39 into 1JLB showed that the terminal methyl group of methoxyformyl on the phenylamine moiety shared the π-alkyl interactions with Trp229, Phe227, and Tyr188 and the alkyl interaction with the Leu234 (Fig. S187b in Supporting Information).
Fig. 3.
Binding modes of 10i into the WT of the HIV-1 RT.
Fig. 4.
Binding modes of 10i into the NNBS of (a) HIV-1 Y181C RT (PDB code: 1JLB); (b) HIV-1 K103N/Y181C RT (PDB code: 5VQY); (c) HIV-1 L100I/K103N RT (PDB code: 2ZE2).
Compound 10i docking into the K103N/Y181C double-mutated RT (5VQY) (Fig. 4b) showed that an H-bond was formed between the Asn103 amide NH2 group and the N-alkoxy oxygen atom, replacing the π-alkyl interaction of the indole ring with Lys103 in WT RT. Meanwhile, the π-π interaction between the pyridine ring and Tyr181 was substituted by a π-sulfur interaction between the indole nucleus and –SH group. Additionally, the pyridine N atom hydrogen-bonded to the phenolic hydroxy proton of Tyr188 and the terminal –NH2 of Lys223, while the pyridine ring maintained the π-π interaction with Tyr188. However, the carbonyl oxygen of methoxyformyl on the phenylamine moiety of CI - 39 hydrogen-bonded to the terminal –NH2 of Lys223 and the phenolic hydroxy proton of Tyr188, while Cys181 maintained the same mode of action as 10i (Fig. S187c in Supporting Information). For the positive control NVP, which is resisted by K103N/Y181C double-mutated RT, the docking simulation (Fig. S185c in Supporting Information) showed no interaction of NVP with Asn103. This suggests that the H-bond between the N-alkoxy oxygen atom and the Asn103 amide NH2 group may play an important role in the effect of 10i, which is supported by the experimental data (Table 4).
In the case of the L100I/K103N double-mutated RT (Figure 6c), the docking exhibited a position exchange between the indole and pyridine ring moieties in the interacting model, and the hydroxy group from isomerization of the acetamide unit in 10i was hydrogen-bonded to the terminal carbamide oxygen of the mutated Asn103. The indole ring began to have a π-π interaction with Tyr188, and the pyridine ring maintained the π-π effect on Tyr181 as well as the acting force with Ile100, in turn which the alkyl-π interaction with the indole nucleus was replaced by an alkyl interaction with the N-butoxy chain. Similarly the docking of CI - 39 displayed the terminal carbamide oxygen of Asn103 hydrogen-bonded to the hydroxy group from isomerization of the acetamide unit (Fig. S187d in Supporting Information). However, the mutated Ile100 exhibited the alkyl interactions with both the methyl group of methoxyformyl and the indole nucleus of CI - 39. As the L100I/K103N double-mutated RT resisted EFV, the docking (Fig. S186d in Supporting Information) showed the absence of interaction between the mutated Ile100 and EFV. This suggests that the interactions between 10i/CI - 39 and the mutated Ile100 are effective on their activities compared to EFV (Table 4).
3. Conclusions
A novel antiviral lead compound CI - 39 was characterized from an aqueous decoction of the traditional Chinese medicine “ban lan gen” (the I. indigotica root), which should have a contribution to the clinical effect of the herbal medicine. Based on the unique structure with potent activity against HIV-1 replication, 57 new derivatives were designed and synthesized, of which 24 were active with EC50 values of 0.06–8.55 μM. Structure-activity relationship of these derivatives was clarified. The biological assays demonstrated that CI - 39 and its derivatives displayed their activities by inhibiting RT DNA polymerase, but no acting on RNase H. The synthetic representatives 10f and 10i exhibited much better inhibitory effects on the six of seven tested resistant strains compared to NVP. Moreover, the two candidates strongly suppressed NNRTI-resistant HIV-1 carrying the RT-K103N/Y181C mutation. Especially 10i was superior to both NVP and EFV against the HIV-1RT-L100I/K103N, which suggested that it has the potential for use in developing a new NNRTI. Additionally, the computational docking results also hinted at possible binding mechanisms of the potent 10i. In summary, this class of derivatives has a good antiviral prospect and deserves further investigation.
4. Experimental
4.1. Chemistry
4.1.1. Isolation and structural determination of CI - 39
General experimental procedures, plant material, extraction, and preliminary fractionation of the extract, see Ref. [20]. Fraction C9 (2.8 g) was subjected to CC over Sephadex LH-20 (CHCl3/MeOH, 1:1) to give C9-1−C9-3, of which C9-2 (1.2 g) was fractionated by RP flash CC (30–70% MeOH in H2O) to give fractions C9-2-1−C9-2-3. Subfraction C-9-2-1 (500 mg) was separated by CC over silica gel eluting with a gradient of increasing MeOH in CHCl3 (1–5%) afforded C-9-2-1-1-C-9-2-1-5, of which C-9-2-1-4 (15.4 mg) was isolated by reversed phase HPLC using a semipreparative C18 column and the mobile phase of 33% MeOH in H2O to yield CI - 39 (2.1 mg).
4.1.1.1. Methyl (1-methoxy-1H-indol-3-yl)acetamidobenzoate (CI - 39)
Yield 0.0000042%. M.p. 95–96 °C; UV (MeOH) λ max (logε) 222(4.81), 252 (4.25), 260 (4.19), 290 (3.99), 300 (3.99) nm; IR (KBr) ν max 3247, 2948, 2842, 1701, 1677, 1585, 1511, 1452, 1410, 1294, 1264, 1092, 1031, 956, 762, 741 cm−1; 1H NMR (acetone-d 6, 500 MHz) data see Table 1; 13C NMR (acetone-d 6, 125 MHz) data, see Table 1. ESIMS m/z 361 [M + Na]+; HR-ESIMS m/z 339.1320 [M + H]+ (Calcd for C19H19N2O4, 339.1339).
4.1.1.2. X-ray crystallography of CI - 39
Molecular formula C19H18N2O4, monoclinic, P21/c, a = 7.449 (3) Å, b = 23.336 (12) Å, c = 10.026 (4) Å, α = γ = 90°, β = 93.84 (8)°, V = 1738.9 (13) Å3, Z = 4, Dcalcd = 1.292 g/cm3, 3391 reflections independent, 2069 reflections observed (|F|2≥ 2σ|F|2), R1 = 0.0521, wR 2 = 0.1246, S = 1.004.
The data were collected on a Rigaku MicroMax 002+ diffractometer with CuKα radiation by using the ω and κ scan technique to a maximum 2θ value of 144.60°. The crystal structure was solved by direct methods by using SHELXS-97, and all non-hydrogen atoms were refined anisotropically using the least-squares method. All hydrogen atoms were positioned by geometric calculations. Crystallographic data have been deposited with the Cambridge Crystallographic Data Center (CCDC 866444). Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: (+44) 1223-336-033; or deposit@ccdc.cam.ac.uk).
4.1.2. Synthesis
All the synthetic starting materials and reagents were purchased from commercial suppliers and used directly without further purification. Column chromatography was performed using silica gel (200–300 mesh) purchased from Qingdao Haiyang Chemical Co., Ltd. Thin-layer chromatography was performed on precoated silica gel F-254 plates and was visualized under UV light. 1H NMR and 13C NMR spectra were recorded on Varian Mercury 300 MHz, 400 MHz or 500 MHz with TMS or solvent peaks as references. Chemical shifts (δ) are reported in parts per million (ppm) and coupling constants (J) are reported in hertz (Hz). The splitting patterns are indicated as: s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublet, m = multiplet. HR-ESIMS data were obtained on an Agilent 6520 Accurate-Mass Q-TOF LCMS spectrometers (Agilent Technologies, Ltd., Santa Clara, CA, USA). Analysis of sample purity was performed on an Agilent HPLC system consisting of 1100 controller, 1100 pump, and 1100 dual λ absorbance detector with an CAPCELL PAK AQ-C18 column (5 μm, 250 × 4.6 mm). HPLC conditions: solvent A: H2O; solvent B: CH3OH; flow rate 1.0 mL/min; compounds were eluted with a gradient of 30% CH3OH in H2O with to 100% CH3OH for 20 min. All tested compounds have a purity ≥95.0%.
4.1.2.1. Synthesis of CI - 39
To a solution of indole-3-acetic acid (1.05 g, 1 equiv.) in dry CH2Cl2 (100 mL) was added EDCI (1.27 g, 1.1 equiv.). The reaction mixture was stirred at room temperature for 1 h, then methyl 2-aminobenzoate (1.0 g, 1.1 equiv.) and DMAP (0.07 g, 0.1 equiv.) were added. The mixture was stirred at room temperature for 3 h; 2 M hydrochloric acid (100 mL) was added and stirred strongly for 10 min. The organic phase was separated and the water phase was extracted with CH2Cl2 (50 mL) twice. The combined organic phase was washed with brine and dried with Na2SO4. Filtration and removal of the solvent at 40 °C under reduced pressure yielded methyl 2-(2-(1H-indol-3-yl)acetamido)benzoate (CI-a, 1.03 g).
To a solution of CI-a (1.03 g, 1 equiv.) in CF3COOH (30 mL) was added Et3SiH (1.2 g, 3 equiv.), stirred at 60 °C for 1 h, followed by recovery of the solvent under reduced pressure to yield a residue. The residue was dissolved in ethyl acetate and washed by saturated Na2CO3 and brine in order, subsequent removal of the solvent under reduced pressure afforded methyl 2-(2-(indolin-3-yl)acetamido)benzoate (CI-b, 0.98 g). To a solution of CI-b (0.98 g, 1 equiv.) in MeOH (50 mL) was added sodium tungstate dehydrate (0.3 g, 0.3 equiv.) at 0 °C, followed by dropwise addition of hydrogen peroxide (30%, 3.5 mL, 10 equiv.) then stirred at 10–15 °C for 1 h. The reaction mixture was partitioned between CH2Cl2 and water, and the organic phase was washed with brine and evaporated, the resulting residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 2:1) to furnish methyl 2-(2-(1-hydroxy-1H-indol-3-yl)acetamido)benzoate (CI-c, 0.69 g). Selectively the CH2Cl2 solution of CI-c (0.69 g) was added a solution of trimethylsilyldiazomethane in n-hexane (6 M, 3.5 mL, 10 equiv), stirred at room temperature overnight, followed by recovery of the organic solvent to give a residue. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate 2:1) to yield CI - 39 (0.49 g, four steps yield 24.2%).
4.1.2.1.1. Methyl 2-(2-(1H-indol-3-yl)acetamido)benzoate (CI-a) [73].
Yield 55.5%. 1H NMR (500 MHz, DMSO‑d 6): δ 11.07 (s, 1H, CONH), 10.59 (s, 1H, NH), 8.41 (d, J = 8.5 Hz, 1H, ArH), 7.84 (dd, J = 2.0, 8.0 Hz, 1H, ArH), 7.58 (t, J = 7.5 Hz, 1H, ArH), 7.51 (d, J = 7.5 Hz, 1H, ArH), 7.38 (m, 2H, ArH), 7.10 (m, 2H, ArH), 6.97 (t, J = 7.5 Hz, 1H, ArH), 3.83 (s, 2H, CH2), 3.64 (s, 3H, CH3). ESIMS m/z 331 [M + Na]+, 344 [M + Cl]-.
4.1.2.1.2. Methyl 2-(2-(indolin-3-yl)acetamido)benzoate (CI-b)
Yield 95.0%. 1H NMR (500 MHz, acetone-d 6): δ 11.00 (s, 1H, CONH), 8.74 (d, J = 8.5 Hz, 1H, ArH), 8.03 (dd, J = 2.0, 8.5 Hz, 1H, ArH), 7.60 (t, J = 8.0 Hz, 1H, ArH), 7.13 (m, 2H, ArH), 6.94 (t, J = 7.5 Hz, 1H, ArH), 6.57 (m, 2H, ArH), 4.89 (s, 1H, NH), 3.90 (s, 3H, CH3), 3.75 (m, 2H, CH2), 3.30 (m, 1H, CH), 2.87 (m, 1H, CH2), 2.67 (m, 1H, CH2); 13C NMR (125 MHz, acetone-d 6): δ 170.9, 169.1, 153.1, 142.3, 135.2, 132.1, 131.6, 128.4, 124.5, 123.2, 120.9, 118.3, 116.0, 109.8, 53.9, 52.8, 43.5, 39.5; HR-ESIMS m/z 311.1398 [M + H]+ (Calcd for C18H19N2O3, 311.1390).
4.1.2.1.3. Methyl 2-(2-(1-hydroxy-1H-indol-3-yl)acetamido)benzoate (CI-c)
Yield 67.5%. 1H NMR (500 MHz, acetone-d 6): δ 10.87 (1H, s, NH), 10.21 (1H, s, OH), 8.71 (1H, d, J = 8.5 Hz, ArH), 7.93 (1H, dd, J = 1.5, 8.0 Hz, ArH), 7.56 (2H, m, ArH), 7.49 (1H, s, ArH), 7.42 (1H, d, J = 8.0 Hz, ArH), 7.17 (1H, t, J = 7.5 Hz, ArH), 7.09 (1H, t, J = 7.5 Hz, ArH), 7.02 (1H, t, J = 7.5 Hz, ArH), 3.86 (2H, s, CH2), 3.74 (3H, s, COOCH3); 13C NMR (150 MHz, acetone-d 6): δ 170.8, 168.2, 142.4, 135.0 (2C), 131.5, 125.8, 124.7, 123.1, 122.8, 120.7, 120.0, 119.6, 116.4, 109.2, 104.2, 52.6, 35.8; HR-ESIMS m/z 325.119 [M + H]+ (Calcd for C18H17N2O4, 325.1183).
4.1.2.2. General procedure for the synthesis of 5, 6 and 10
The first two steps of general procedure for the synthesis of intermediates 2, 3, 7 and 8 are same as that of preparing CI-a, CI-b. To a solution of 3 (1 equiv.) in MeOH was added m-CPBA (2 equiv.), stirred at room temperature for 2 h, then concentrated under reduced pressure to yield a residue, which was dissolved in CH2Cl2 and washed successively by saturated Na2CO3 and brine. The CH2Cl2 solution was evaporated and the resulting residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 2:1) to furnish 4. Similarly, 9 was obtained as same as the procedure of prepare CI-c. Selectively the CH2Cl2 solution of 4 or 9 was added to a solution of trimethylsilyldiazomethane in n-hexane (6 M, 10 equiv) or the alkyl halide (1.5 equiv.), stirred at room temperature overnight or for 2 h in DMF with K2CO3 (2 equiv.), followed by partition between CH2Cl2 and water, then dry with Na2SO4 and recovery of the organic solvent or to give a residue. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate 2:1) to yield 5, 6, and 10.
4.1.2.2.1. 2-(1-Methoxy-1H-indol-3-yl)-N-phenylacetamide (5a)
Four steps yield 29.6%. 1H NMR (400 MHz, acetone-d 6): δ 9.16 (s, 1H, CONH), 7.64 (m, 3H, ArH), 7.46 (s, 1H, ArH), 7.42 (d, J = 8.4 Hz, 1H, ArH), 7.23 (m, 3H, ArH), 7.04 (m, 2H, ArH), 4.05 (s, 3H, OCH3), 3.79 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 140.4, 133.4, 129.4 (2C), 124.9, 124.1, 123.4, 123.2, 120.4, 120.2 (2C), 120.1, 109.0, 106.6, 66.1, 34.6; HR-ESIMS m/z 281.1287 [M + H]+ (Calcd for C17H17N2O2, 281.1285).
4.1.2.2.2. 2-(1-Methoxy-1H-indol-3-yl)-N-(o-tolyl)acetamide (5b)
Four steps yield 20.0%. 1H NMR (400 MHz, acetone-d 6): δ 8.33 (s, 1H, CONH), 7.72 (d, J = 8.0 Hz, 1H, ArH), 7.68 (d, J = 8.0 Hz, 1H, ArH), 7.54 (s, 1H, ArH), 7.45 (d, J = 8.4 Hz, 1H, ArH), 7.24 (t, J = 7.6 Hz, 1H, ArH), 7.10 (m, 3H, ArH), 6.99 (t, J = 7.6 Hz, 1H, ArH), 4.10 (s, 3H, OCH3), 3.84 (s, 2H, CH2), 2.01 (s, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.7, 137.6, 133.4, 131.0, 130.7, 126.9, 125.4, 124.8, 124.1, 123.6, 123.3, 120.6, 120.1, 109.0, 106.6, 66.2, 34.3, 17.7; HR- ESIMS m/z 295.1447 [M + H]+ (Calcd for C18H19N2O2, 295.1441).
4.1.2.2.3. 2-(1-Methoxy-1H-indol-3-yl)-N-(p-tolyl)acetamide (5c)
Four steps yield 27.8%. 1H NMR (400 MHz, acetone-d 6): δ 9.11 (s, 1H, CONH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.51 (d, J = 8.4 Hz, 2H, ArH), 7.44 (s, 1H, ArH), 7.41 (d, J = 8.4 Hz, 1H, ArH), 7.21 (t, J = 8.0 Hz, 1H, ArH), 7.06 (t, 3H, ArH), 4.06 (s, 3H, OCH3), 3.77 (s, 2H, CH2), 2.24 (s, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.7, 137.8, 133.3, 129.8 (2C), 124.9, 123.4, 123.1, 120.4, 120.2, 108.9, 106.7, 66.1, 34.6, 20.7; HR-ESIMS m/z 295.145 [M + H]+ (Calcd for C18H19N2O2, 295.1441).
4.1.2.2.4. N-(2-Ethylphenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5d)
Four steps yield 27.8%. 1H NMR (400 MHz, acetone-d 6): δ 8.20 (s, 1H, CONH), 7.76 (d, J = 8.0 Hz, 1H, ArH), 7.68 (d, J = 8.0 Hz, 1H, ArH), 7.57 (s, 1H, ArH), 7.46 (d, J = 7.6 Hz, 1H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.11 (m, 3H, ArH), 7.03 (t, J = 6.8 Hz, 1H, ArH), 4.12 (s, 3H, OCH3), 3.84 (s, 2H, CH2), 2.33 (q, J = 7.6, 14.8 Hz, 2H, CH2), 0.85 (t, 3H, J = 7.6 Hz, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.8, 136.8, 136.4, 133.4, 129.4, 126.9, 125.7, 124.7, 124.4, 123.7, 123.5, 120.7, 120.1, 109.1, 106.5, 66.3, 34.4, 24.7, 14.3; HR-ESIMS m/z 309.1601 [M + H]+ (Calcd for C19H21N2O2, 309.1598).
4.1.2.2.5. Methyl 3-[2-(1-methoxy-1H-indol-3-yl)acetamido]benzoate (5e)
Four steps yield 21.4%. 1H NMR (400 MHz, acetone-d 6): δ 9.37 (s, 1H, CONH), 8.29 (s, 1H, ArH), 7.89 (d, J = 8.0 Hz, 1H, ArH), 7.67 (d, J = 7.6 Hz, 2H, ArH), 7.48 (s, 1H, ArH), 7.40 (m, 2H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 4.09 (s, 3H, OCH3), 3.84 (s, 3H, COOCH3), 3.83 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.2, 167.0, 140.6, 133.4, 131.6, 129.7, 124.9, 124.86, 124.4, 123.5, 123.2, 120.9, 120.5, 120.1, 109.0, 106.3, 66.2, 52.3, 34.6; HR-ESIMS m/z 339.1347 [M + H]+ (Calcd for C19H19N2O4, 339.1339).
4.1.2.2.6. Methyl 4-[2-(1-methoxy-1H-indol-3-yl)acetamido]benzoate (5f)
Four steps yield 24.7%. 1H NMR (400 MHz, acetone-d 6): δ 9.49 (s, 1H, CONH), 7.92 (d, 2H, J = 8.4 Hz, ArH), 7.76 (d, J = 8.4 Hz, 2H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.46 (s, 1H, ArH), 7.41 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.07 (t, J = 7.6, 1H, ArH), 4.10 (s, 3H, OCH3), 3.84 (s, 2H, CH2), 3.83 (s, 3H, COOCH3); 13C NMR (100 MHz, acetone-d 6): δ 170.5, 166.7, 144.5, 133.3, 131.1 (2C), 125.6, 124.8, 123.5, 123.2, 120.5, 120.1, 119.3 (2C), 109.0, 106.1, 66.1, 52.0, 34.7; HR-ESIMS m/z 339.1344 [M + H]+ (Calcd for C19H19N2O4, 339.1339).
4.1.2.2.7. Ethyl 2-[2-(1-methoxy-1H-indol-3-yl)acetamido]benzoate (5g)
Four steps yield 20.0%. 1H NMR (400 MHz, acetone-d 6): δ 11.00 (s, 1H, CONH), 8.76 (d, J = 8.4 Hz, 1H, ArH), 7.92 (d, J = 8.0 Hz, 1H, ArH), 7.60 (s, 1H, ArH), 7.59 (d, J = 9.2 Hz, 1H, ArH), 7.53 (t, J = 8.0 Hz, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (m, 2H, ArH), 4.180 (m, 5H, CH2, OCH3), 3.87 (s, 2H, CH2), 1.25 (t, J = 6.8 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 170.7, 168.2, 142.4, 134.9, 133.3, 131.5, 124.8, 124.2, 123.3, 123.0, 120.6, 120.5, 119.8, 116.2, 109.0, 105.1, 66.4, 61.9, 35.7, 14.3; HR-ESIMS m/z 353.1504 [M + H]+ (Calcd for C20H21N2O4, 353.1496).
4.1.2.2.8. 2-(1-Methoxy-1H-indol-3-yl)-N-(4-nitrophenyl)acetamide (5h)
Four steps yield 25.9%. 1H NMR (400 MHz, acetone-d 6): δ 9.76 (s, 1H, CONH), 8.17 (d, J = 9.2 Hz, 2H, ArH), 7.88 (d, J = 8.8 Hz, 2H, ArH), 7.64 (d, J = 7.6 Hz, 1H, ArH), 7.49 (s, 1H, ArH), 7.42 (d, J = 7.6 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.07 (t, J = 7.2 Hz, 1H, ArH), 4.09 (s, 3H, OCH3), 3.88 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.8, 146.3, 143.8, 133.3, 125.5 (2C), 124.8, 123.6, 123.3, 120.5, 120.1, 119.7 (2C), 109.0, 105.8, 66.2, 34.7; HR-ESIMS m/z 326.1144 [M + H]+ (Calcd for C17H16N3O4, 326.1135).
4.1.2.2.9. N-(2-Fluorophenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5i)
Four steps yield 18.5%. 1H NMR (400 MHz, acetone-d 6): δ 8.83 (s, 1H, CONH), 8.22 (t, J = 8.0 Hz, 1H, ArH), 7.68 (d, J = 8.0 Hz, 1H, ArH), 7.52 (s, 1H, ArH), 7.44 (d, J = 8.0 Hz, 1H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 7.09 (m, 4H, ArH), 4.09 (s, 3H, OCH3), 3.91 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.1, 133.4, 125.2, 125.14, 125.09, 125.06, 124.8, 123.5, 123.4, 123.3, 120.5, 120.1, 115.8, 115.6, 109.0, 106.3, 66.2, 34.3; HR-ESIMS m/z 299.1193 [M + H]+ (Calcd for C17H16N2O2F, 299.1190).
4.1.2.2.10. N-(2-Chlorophenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5j)
Four steps yield 22.0%. 1H NMR (400 MHz, acetone-d 6): δ 8.46 (s, 1H, CONH), 8.29 (d, J = 8.0 Hz, 1H, ArH), 7.67 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 7.05 (t, J = 8.0 Hz, 1H, ArH), 4.12 (s, 3H, OCH3), 3.925 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.4, 129.9, 128.3, 125.5, 124.7, 123.9, 123.5, 122.9 (2C), 120.8, 120.0, 109.1, 105.8, 66.3, 34.5; HR-ESIMS m/z 315.0905 [M + H]+ (Calcd for C17H16N2O2Cl, 315.0895).
4.1.2.2.11. N-(3-Chlorophenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5k)
Four steps yield 23.1%. 1H NMR (400 MHz, acetone-d 6): δ 9.33 (s, 1H, CONH), 7.89 (s, 1H, ArH), 7.64 (d, J = 8.0 Hz, 1H, ArH), 7.44 (m, 3H, ArH), 7.26 (t, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 8.0 Hz, 1H, ArH), 7.06 (m, 2H, ArH), 4.09 (s, 3H, OCH3), 3.80 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.3, 141.7, 134.6, 133.3, 130.9, 124.8, 123.9, 123.5, 123.2, 120.5, 120.1, 119.9, 118.3, 109.0, 106.2, 66.2, 34.6; HR-ESIMS m/z 315.0897 [M + H]+ (Calcd for C17H16N2O2Cl, 315.0895).
4.1.2.2.12. N-(4-Chlorophenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5l)
Four steps yield 20.4%. 1H NMR (400 MHz, acetone-d 6): δ 9.30 (s, 1H, CONH), 7.65 (m, 3H, ArH), 7.46 (s, 1H, ArH), 7.41 (d, J = 8.4 Hz, 1H, ArH), 7.27 (d, J = 8.4 Hz, 2H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 4.08 (s, 3H, OCH3), 3.79 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.1, 139.2, 133.3, 129.3 (2C), 128.4, 124.8, 123.5, 123.2, 121.6 (2C), 120.5, 120.1, 109.0, 106.3, 66.2, 34.6; HR-ESIMS m/z 315.0905 [M + H]+ (Calcd for C17H16N2O2Cl, 315.0895).
4.1.2.2.13. N-(2-Bromophenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5m)
Four steps yield 11.1%. 1H NMR (400 MHz, acetone-d 6): δ 8.32 (s, 1H, CONH), 8.27 (d, J = 8.0 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.64 (s, 1H, ArH), 7.49 (d, J = 7.6 Hz, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.32 (t, J = 8.0 Hz, 1H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 6.99 (t, J = 8.0 Hz, 1H, ArH), 4.13 (s, 3H, OCH3), 3.91 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 137.2, 133.4, 133.2, 129.0, 126.0, 124.7, 124.0, 123.6, 122.9, 120.9, 120.0, 114.2, 109.1, 105.6, 66.4, 34.6; HR-ESIMS m/z 359.0403 [M + H]+ (Calcd for C17H16N2O2Br, 359.0390).
4.1.2.2.14. N-(2-Iodophenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5n)
Four steps yield 10.3%. 1H NMR (400 MHz, acetone-d 6): δ 8.17 (d, J = 8.4 Hz, 1H, ArH), 8.13 (s, 1H, CONH), 7.72 (d, J = 7.6 Hz, 1H, ArH), 7.65 (m, 2H, ArH), 7.47 (d, J = 8.4 Hz, 1H, ArH), 7.33 (t, J = 8.0 Hz, 1H, ArH), 7.25 (t, J = 8.0 Hz, 1H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 6.83 (t, J = 7.6 Hz, 1H, ArH), 4.14 (s, 3H, OCH3), 3.89 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 139.8 (2C), 133.5, 129.7, 126.7, 124.7, 124.2, 123.6, 122.7 (2C), 120.9, 120.1, 109.2, 105.4, 66.5, 34.6; HR-ESIMS m/z 407.0269 [M + H]+ (Calcd for C17H16IN2O2, 407.0251).
4.1.2.2.15. 2-(1-Methoxy-1H-indol-3-yl)-N-(2-methoxyphenyl)acetamide (5o)
Four steps yield 35.2%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.33 (d, J = 7.6 Hz, 1H, ArH), 7.67 (d, J = 7.6 Hz, 1H, ArH), 7.56 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.24 (t, J = 7.6 Hz, 1H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 6.96 (t, J = 8.0 Hz, 1H, ArH), 6.87 (m, 2H, ArH), 4.13 (s, 3H, OCH3), 3.86 (s, 2H, CH2), 3.64 (s, 3H, OCH3); 13C NMR (100 MHz, acetone-d 6): δ 169.5, 149.1, 133.4, 129.1, 124.7, 124.2, 123.7, 123.4, 121.3, 120.7, 120.13, 120.09, 111.2, 109.1, 106.4, 66.3, 56.0, 34.8; HR-ESIMS m/z 311.1397 [M + H]+ (Calcd for C18H19N2O3, 311.1390).
4.1.2.2.16. 2-(1-Methoxy-1H-indol-3-yl)-N-(3-methoxyphenyl)acetamide (5p)
Four steps yield 34.6%. 1H NMR (400 MHz, acetone-d 6): δ 9.30 (s, 1H, CONH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.46 (s, 1H, ArH), 7.41 (d, J = 6.8 Hz, 2H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.13 (m, 2H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 6.59 (d, J = 6.8 Hz, 1H, ArH), 4.08 (s, 3H, OCH3), 3.78 (s, 2H, CH2), 3.72 (s, 3H, OCH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 160.9, 141.6, 133.3, 130.1, 124.9, 123.4, 123.1, 120.4, 120.2, 112.3, 109.5, 108.9, 106.6, 105.9, 66.1, 55.4, 34.6; HR-ESIMS m/z 311.1399 [M + H]+ (Calcd for C18H19N2O3, 311.1390).
4.1.2.2.17. 2-(1-Methoxy-1H-indol-3-yl)-N-(4-methoxyphenyl)acetamide (5q)
Four steps yield 32.9%. 1H NMR (400 MHz, acetone-d 6): δ 9.02 (s, 1H, CONH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.52 (d, J = 8.8 Hz, 2H, ArH), 7.45 (s, 1H, ArH), 7.41 (d, J = 8.0 Hz, 1H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 6.82 (d, J = 8.8 Hz, 2H, ArH), 4.08 (s, 3H, OCH3), 3.75 (s, 2H, CH2), 3.73 (s, 3H, OCH3); 13C NMR (100 MHz, acetone-d 6): δ 169.5, 156.7, 133.5, 133.4, 124.9, 123.4, 123.2, 121.7 (2C), 120.4, 120.2, 114.5 (2C), 108.9, 106.7, 66.1, 55.6, 34.5; HR-ESIMS m/z 311.1398 [M + H]+ (Calcd for C18H19N2O3, 311.1390).
4.1.2.2.18. N-(3,4-Dimethylphenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5r)
Four steps yield 26.9%. 1H NMR (400 MHz, acetone-d 6): δ 8.99 (s, 1H, CONH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.45 (s, 1H, ArH), 7.41 (d, J = 8.0 Hz, 1H, ArH), 7.38 (s, 1H, ArH), 7.35 (d, J = 8.0 Hz, 1H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 6.99 (d, J = 8.0 Hz, 1H, ArH), 4.08 (s, 3H, OCH3), 3.76 (s, 2H, CH2), 2.16 (m, 6H, CH3 × 2); 13C NMR (100 MHz, acetone-d 6): δ 169.6, 138.1, 137.3, 133.4, 132.0, 130.4, 124.9, 123.4, 123.2, 121.4, 120.4, 120.2, 117.7, 108.9, 106.7, 66.1, 34.6, 19.9, 19.1; HR-ESIMS m/z 309.1601 [M + H]+ (Calcd for C19H21N2O2, 309.1598).
4.1.2.2.19. N-(3,5-Dimethylphenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5s)
Four steps yield 29.9%. 1H NMR (400 MHz, acetone-d 6): δ 9.00 (s, 1H, CONH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.46 (s, 1H, ArH), 7.41 (d, J = 8.0 Hz, 1H, ArH), 7.25 (s, 2H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 6.66 (s, 1H, ArH), 4.08 (s, 3H, OCH3), 3.77 (s, 2H, CH2), 2.20 (s, 6H, CH3 × 2); 13C NMR (100 MHz, acetone-d 6): δ 169.7, 140.2, 138.8 (2C), 133.4, 125.7, 124.9, 123.4, 123.2, 120.4, 120.2, 117.9 (2C), 108.9, 106.7, 66.1, 34.7, 21.4 (2C); HR-ESIMS m/z 309.1608 [M + H]+ (Calcd for C19H21N2O2, 309.1598).
4.1.2.2.20. N-(3,5-Dimethoxyphenyl)-2-(1-methoxy-1H-indol-3-yl)acetamide (5t)
Four steps yield 25.4%. 1H NMR (400 MHz, acetone-d 6): δ 9.19 (s, 1H, CONH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.44 (s, 1H, ArH), 7.41 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 6.92 (s, 2H, ArH), 6.20 (s, 1H, ArH), 4.07 (s, 3H, OCH3), 3.78 (s, 2H, CH2), 3.70 (s, 6H, OCH3 × 2); 13C NMR (100 MHz, acetone-d 6): δ 170.0, 161.9 (2C), 141.9, 133.3, 124.8, 123.4, 123.2, 120.4, 120.2, 108.9, 106.5, 98.4 (2C), 96.2, 66.1, 55.4 (2C), 34.7; HR-ESIMS m/z 341.1506 [M + H]+ (Calcd for C19H21N2O4, 341.1496).
4.1.2.2.21. 2-(1-Methoxy-1H-indol-3-yl)-N-(3,4,5-trimethoxyphenyl)acetamide (5u)
Four steps yield 23.9%. 1H NMR (400 MHz, acetone-d 6): δ 9.12 (s, 1H, CONH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.45 (s, 1H, ArH), 7.42 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 7.03 (s, 2H, ArH), 4.09 (s, 3H, OCH3), 3.78 (s, 2H, CH2), 3.72 (s, 6H, OCH3 × 2), 3.65 (s, 3H, OCH3); 13C NMR (100 MHz, acetone-d 6): δ 169.8, 154.2 (2C), 136.4, 133.4, 124.9, 123.4, 123.2, 120.4, 120.2, 109.0, 106.6, 98.0 (2C), 66.2, 60.5, 56.2 (2C), 34.7; HR-ESIMS m/z 371.1613 [M + H]+ (Calcd for C20H23N2O5, 371.1601).
4.1.2.2.22. Methyl 2-[3-(1-methoxy-1H-indol-3-yl)propanamido]benzoate (5v)
Four steps yield 11.7%. 1H NMR (400 MHz, acetone-d 6): δ 10.95 (s, 1H, CONH), 8.72 (d, J = 8.8 Hz, 1H, ArH), 7.99 (d, J = 8.0 Hz, 1H, ArH), 7.64 (d, J = 7.6 Hz, 1H, ArH), 7.58 (t, J = 7.6 Hz, 1H, ArH), 7.38 (d, J = 8.4 Hz, 1H, ArH), 7.35 (s, 1H, ArH), 7.19 (t, J = 7.6 Hz, 1H, ArH), 7.11 (t, J = 7.6 Hz, 1H, ArH), 7.05 (t, J = 7.6 Hz, 1H, ArH), 4.03 (s, 3H, OCH3), 3.89 (s, 3H, COOCH3), 3.15 (t, J = 7.6 Hz, 2H, CH2), 2.83 (t, J = 7.6 Hz, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 171.6, 169.1, 142.5, 135.2, 133.6, 131.6, 124.7, 123.1 (2C), 122.1, 120.9, 120.2, 119.8, 115.9, 111.9, 109.0, 65.9, 52.8, 39.5, 21.4; HR-ESIMS m/z 353.1508 [M + H]+ (Calcd for C20H21N2O4, 353.1496).
4.1.2.2.23. Methyl 2-(4-(1-methoxy-1H-indol-3-yl)butanamido)benzoate (5w)
Four steps yield 9.2%. 1H NMR (500 MHz, acetone-d 6): δ 10.96 (s, 1H, CONH), 8.72 (d, J = 8.5 Hz, 1H, ArH), 8.01 (dd, J = 1.5, 8.0 Hz, 1H, ArH), 7.59 (m, 2H, ArH), 7.39 (d, J = 8.0 Hz, 1H, ArH), 7.32 (s, 1H, ArH), 7.18 (t, J = 7.5 Hz, 1H, ArH), 7.12 (t, J = 7.5 Hz, 1H, ArH), 7.04 (t, J = 7.5 Hz, 1H, ArH), 4.06 (s, 3H, OCH3), 3.91 (s, 3H, COOCH3), 2.83 (t, J = 7.5 Hz, 2H, CH2), 2.52 (t, J = 7.0 Hz, 2H, CH2), 2.11 (m, 2H, CH2); 13C NMR (125 MHz, acetone-d 6): δ 172.0, 169.2, 142.6, 135.2, 133.8, 131.6, 124.9, 123.0 (2C), 122.1, 120.8, 120.1, 119.9, 115.8, 112.6, 109.0, 65.8, 52.8, 38.3, 26.6, 24.9; HR-ESIMS m/z 367.166 [M + H]+ (Calcd for C21H23N2O4, 367.1652).
4.1.2.2.24. Methyl 2-(2-(1-ethoxy-1H-indol-3-yl)acetamido)benzoate (6a)
Four steps yield 24.1%. 1H NMR (400 MHz, acetone-d 6): δ 10.93 (s, 1H, CONH), 8.75 (d, J = 8.4 Hz, 1H, ArH), 7.90 (d, J = 8.4 Hz, 1H, ArH), 7.56 (m, 3H, ArH), 7.46 (d, J = 8.4 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (m, 2H, ArH), 4.43 (m, 2H, OCH2), 3.87 (s, 2H, CH2), 3.71 (s, 3H, COOCH3), 1.43 (t, J = 6.8 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 170.2, 168.0, 141.7, 134.4, 133.5, 130.9, 124.4, 124.1, 122.6, 122.5, 120.0, 119.9, 119.1, 115.4, 108.7, 104.2, 74.3, 51.9, 35.1, 13.6; HR-ESIMS m/z 353.1502 [M + H]+ (Calcd for C20H21N2O4, 353.1496).
4.1.2.2.25. Methyl 2-(2-(1-propoxy-1H-indol-3-yl)acetamido)benzoate (6b)
Four steps yield 23.8%. 1H NMR (400 MHz, acetone-d 6): δ 10.92 (s, 1H, CONH), 8.75 (d, J = 8.4 Hz, 1H, ArH), 7.90 (d, J = 8.4 Hz, 1H, ArH), 7.56 (m, 3H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (m, 2H, ArH), 4.34 (t, J = 6.4 Hz, 2H, OCH2), 3.87 (s, 2H, CH2), 3.72 (s, 3H, COOCH3), 1.86 (m, 2H, CH2), 1.10 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 170.8, 168.6, 142.3, 135.0, 133.9, 131.5, 124.9, 124.7, 123.2, 123.1, 120.6, 120.5, 119.7, 116.0, 109.2, 104.9, 80.8, 52.5, 35.7, 22.3, 10.6; HR-ESIMS m/z 367.1664 [M + H]+ (Calcd for C21H23N2O4, 367.1652).
4.1.2.2.26. N-(2-Chlorophenyl)-2-(1-ethoxy-1H-indol-3-yl)acetamide (6c)
Four steps yield 25.3%. 1H NMR (400 MHz, acetone-d 6): δ 8.44 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.67 (d, J = 8.0 Hz, 1H, ArH), 7.60 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.32 (d, J = 8.0 Hz, 1H, ArH), 7.25 (m, 2H, ArH), 7.09 (t, J = 7.6 Hz, 1H, ArH), 7.04 (t, J = 7.6 Hz, 1H, ArH), 4.35 (m, 2H, ArH), 3.93 (s, 2H, CH2), 1.38 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.0, 134.0, 129.8, 125.5, 124.7, 124.5, 123.4 (2C), 122.8 (2C), 120.7, 119.9, 109.3, 105.4, 74.7, 34.5, 14.1; HR-ESIMS m/z 329.106 [M + H]+ (Calcd for C18H18N2O2Cl, 329.1051).
4.1.2.2.27. N-(2-Chlorophenyl)-2-(1-propoxy-1H-indol-3-yl)acetamide (6d)
Four steps yield 24.9%. 1H NMR (400 MHz, acetone-d 6): δ 8.44 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.67 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.09 (t, J = 7.6 Hz, 1H, ArH), 7.04 (t, J = 7.6 Hz, 1H, ArH), 4.26 (t, J = 6.8 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.81 (m, 2H, CH2), 1.07 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.3, 125.5 (2C), 124.6, 123.5 (2C), 122.8, 120.7, 119.9, 109.3, 105.5, 80.7, 34.6, 22.3, 10.5; HR-ESIMS m/z 343.1221 [M + H]+ (Calcd for C19H20N2O2Cl, 343.1208).
4.1.2.2.28. 2-(1-Butoxy-1H-indol-3-yl)-N-(2-chlorophenyl)acetamide (6e)
Four steps yield 26.2%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.09 (t, J = 7.6 Hz, 1H, ArH), 7.04 (t, J = 7.6 Hz, 1H, ArH), 4.30 (t, J = 6.4 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.78 (m, 2H, CH2), 1.56 (m, 2H, CH2), 0.98 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.4, 125.5 (2C), 124.6, 123.5 (2C), 122.8, 120.7, 120.0, 109.2, 105.5, 79.0, 34.6, 31.0, 19.7, 14.1; HR-ESIMS m/z 357.1373 [M + H]+ (Calcd for C20H22N2O2Cl, 357.1364).
4.1.2.2.29. N-(2-Chlorophenyl)-2-(1-(pentyloxy)-1H-indol-3-yl)acetamide (6f)
Four steps yield 24.7%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.07 (m, 2H, ArH), 4.30 (t, J = 6.4 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.80 (m, 2H, CH2), 1.51 (m, 2H, CH2), 1.41 (m, 2H, CH2), 0.93 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.4, 125.5 (2C), 124.6, 123.5 (2C), 122.8, 120.7, 120.0, 109.3, 105.5, 79.3, 34.6, 28.70, 28.68, 23.1, 14,2; HR-ESIMS m/z 371.1528 [M + H]+ (Calcd for C21H24N2O2Cl, 371.1521).
4.1.2.2.30. N-(2-Chlorophenyl)-2-(1-(hexyloxy)-1H-indol-3-yl)acetamide (6g)
Four steps yield 23.0%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.09 (t, J = 7.6 Hz, 1H, ArH), 7.04 (t, J = 7.6 Hz, 1H, ArH), 4.30 (t, J = 6.4 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.80 (m, 2H, CH2), 1.53 (m, 2H, CH2), 1.35 (m, 4H, CH2), 0.90 (t, J = 6.4 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.4, 125.5 (2C), 124.6, 123.5 (2C), 122.8, 120.7, 120.0, 109.3, 105.5, 79.3, 34.6, 32.3, 29.0, 26.2, 23.2, 14.2; HR-ESIMS m/z 385.1678 [M + H]+ (Calcd for C22H26N2O2Cl, 385.1677).
4.1.2.2.31. N-(2-Chlorophenyl)-2-(1-(heptyloxy)-1H-indol-3-yl)acetamide (6h)
Four steps yield 24.3%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.07 (m, 2H, ArH), 4.30 (t, J = 6.4 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.81 (m, 2H, CH2), 1.53 (m, 2H, CH2), 1.34 (m, 6H, CH2), 0.88 (t, J = 6.0 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.4, 125.5 (2C), 124.6, 123.5 (2C), 122.8, 120.7, 120.0, 109.3, 105.5, 79.3, 34.6, 32.4, 29.8, 29.0, 26.5, 23.2, 14.3; HR-ESIMS m/z 399.184 [M + H]+ (Calcd for C23H28N2O2Cl, 399.1834).
4.1.2.2.32. N-(2-Chlorophenyl)-2-(1-(octyloxy)-1H-indol-3-yl)acetamide (6i)
Four steps yield 24.4%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.07 (m, 2H, ArH), 4.31 (t, J = 6.4 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.81 (m, 2H, CH2), 1.53 (m, 2H, CH2), 1.32 (m, 8H, CH2), 0.88 (t, J = 6.4 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.4, 125.5 (2C), 124.6, 123.5 (2C), 122.8, 120.7, 120.0, 109.3, 105.5, 79.3, 34.6, 32.5, 30.1, 29.9, 29.0, 26.5, 23.3, 14.3; HR-ESIMS m/z 413.1996 [M + H]+ (Calcd for C24H30N2O2Cl, 413.1990).
4.1.2.2.33. N-(2-Chlorophenyl)-2-(1-(nonyloxy)-1H-indol-3-yl)acetamide (6j)
Four steps yield 25.8%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.09 (t, J = 7.6 Hz, 1H, ArH), 7.05 (t, J = 7.6 Hz, 1H, ArH), 4.30 (t, J = 6.4 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.80 (m, 2H, CH2), 1.53 (m, 2H, CH2), 1.33 (m, 10H, CH2), 0.87 (t, J = 6.4 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.4, 125.5 (2C), 124.6, 123.5 (2C), 122.7, 120.7, 120.0, 109.3, 105.5, 79.3, 34.6, 32.5, 30.2, 30.1, 29.9, 29.0, 26.5, 23.3, 14.3; HR-ESIMS m/z 427.2158 [M + H]+ (Calcd for C25H32N2O2Cl, 427.2147).
4.1.2.2.34. N-(2-Chlorophenyl)-2-(1-(decyloxy)-1H-indol-3-yl)acetamide (6k)
Four steps yield 26.1%. 1H NMR (400 MHz, acetone-d 6): δ 8.42 (s, 1H, CONH), 8.30 (d, J = 8.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.26 (m, 2H, ArH), 7.07 (m, 2H, ArH), 4.30 (t, J = 6.4 Hz, 2H, OCH2), 3.92 (s, 2H, CH2), 1.81 (m, 2H, CH2), 1.53 (m, 2H, CH2), 1.32 (m, 12H, CH2), 0.87 (t, J = 6.4 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 136.1, 133.9, 129.9, 128.4, 125.5 (2C), 124.6, 123.5 (2C), 122.7, 120.7, 120.0, 109.3, 105.5, 79.3, 34.6, 32.6, 30.2 (2C), 30.1, 30.0, 29.0, 26.5, 23.3, 14.3; HR-ESIMS m/z 441.2309 [M + H]+ (Calcd for C26H34N2O2Cl, 441.2303).
4.1.2.2.35. 2-(1-(Benzyloxy)-1H-indol-3-yl)-N-(2-chlorophenyl)acetamide (6l)
Four steps yield 22.9%. 1H NMR (300 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.31 (dd, J = 1.2, 8.4 Hz, 1H, ArH), 7.35 (m, 6H, ArH), 7.07 (m, 2H, ArH), 5.28 (2H, s, CH2), 3.90 (s, 2H, CH2); 13C NMR (75 MHz, acetone-d 6): δ 169.9, 136.0, 135.8, 134.0, 130.5 (2C), 129.81, 129.78, 129.7, 129.3 (2C), 128.3, 125.5, 124.8, 124.5, 123.4, 122.9, 120.7, 119.9, 109.4, 105.5, 80.9, 34.5; HR-ESIMS m/z 391.1215 [M + H]+ (Calcd for C23H20N2O2Cl, 391.1208).
4.1.2.2.36. N-(2-Chlorophenyl)-2-(1-phenethoxy-1H-indol-3-yl)acetamide (6m)
Four steps yield 22.6%. 1H NMR (400 MHz, acetone-d 6): δ 8.43 (s, 1H, CONH), 8.28 (d, J = 8.4 Hz, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.57 (s, 1H, ArH), 7.31 (m, 8H, ArH), 7.18 (t, 1H, ArH), 7.06 (m, 2H, ArH), 4.54 (t, J = 6.8 Hz, 2H, CH2), 3.91 (s, 2H, CH2), 3.14 (t, J = 6.8 Hz, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 138.8, 136.1, 133.9, 129.9 (2C), 129.8, 129.3 (2C), 128.4, 127.4, 125.5, 124.61, 124.58, 123.5, 122.9 (2C), 120.8, 119.9, 109.3, 105.7, 79.7, 35.3, 34.5; HR-ESIMS m/z 405.1345 [M + H]+ (Calcd for C24H22N2O2Cl, 405.1364).
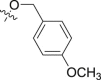
4.1.2.2.37. N-(2-Chlorophenyl)-2-{1-[(4-methoxybenzyl)oxy]-1H-indol-3-yl}acetamide (6n)
Four steps yield 28.9%. 1H NMR (400 MHz, acetone-d 6): δ 8.39 (s, 1H, CONH), 8.28 (d, J = 8.0 Hz, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.42 (m, 4H, ArH), 7.34 (d, J = 8.0 Hz, 1H, ArH), 7.28 (t, J = 8.0 Hz, 1H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.07 (m, 2H, ArH), 6.91 (d, J = 8.4 Hz, 2H, ArH), 5.22 (s, 2H, CH2), 3.88 (s, 2H, CH2), 3.76 (s, 3H, OCH3); 13C NMR (100 MHz, acetone-d 6): δ 169.9, 161.3, 136.1, 134.0, 132.3 (2C), 129.9, 128.3, 127.9, 125.5, 125.0, 124.6 (2C), 123.4, 122.9, 120.7, 119.9, 114.7 (2C), 109.5, 105.4, 80.6, 55.5, 34.5; HR-ESIMS m/z 421.1312 [M + H]+ (Calcd for C24H22N2O3Cl, 421.1313).
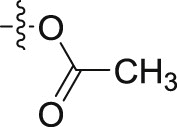
4.1.2.2.38. 3-(2-((2-Chlorophenyl)amino)-2-oxoethyl)-1H-indol-1-yl acetate (6o)
Four steps yield 23.4%. 1H NMR (400 MHz, acetone-d 6): δ 8.52 (s, 1H, CONH), 8.28 (d, J = 8.0 Hz, 1H, ArH), 7.69 (d, J = 8.4 Hz, 1H, ArH), 7.51 (s, 1H, ArH), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.26 (m, 2H, ArH), 7.14 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 3.95 (s, 2H, CH2), 2.42 (s, 3H, COCH3); 13C NMR (100 MHz, acetone-d 6): δ 169.7, 169.4, 136.1, 135.3, 129.9, 128.3, 125.8, 125.6, 125.1, 124.0, 123.0, 121.3, 120.0, 109.4, 107.4, 34.4, 18.0; HR-ESIMS m/z 343.0838 [M + H]+ (Calcd for C18H16N2O3Cl, 343.0844).
4.1.2.2.39. 3-(2-((2-Chlorophenyl)amino)-2-oxoethyl)-1H-indol-1-yl benzoate (6p)
Four steps yield 21.9%. 1H NMR (400 MHz, acetone-d 6): δ 8.60 (s, 1H, CONH), 8.29 (d, J = 8.0 Hz, 1H, ArH), 8.240 (d, J = 7.6 Hz, 2H, ArH), 7.81 (t, J = 7.2 Hz, 1H, ArH), 7.75 (d, J = 8.0 Hz, 1H, ArH), 7.66 (m, 3H, ArH), 7.38 (m, 2H, ArH), 7.28 (m, 2H, ArH), 7.18 (t, J = 7.6 Hz, 1H, ArH), 7.07 (t, J = 7.6 Hz, 1H, ArH), 4.01 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 169.7, 165.3, 136.1, 135.8, 135.6, 130.9 (2C), 130.0 (2C), 129.9, 128.3, 127.4, 126.3, 125.6, 125.4, 124.2, 123.1 (2C), 121.6, 120.2, 109.6, 108.0, 34.4; HR-ESIMS m/z 405.1010 [M + H]+ (Calcd for C23H18N2O3Cl, 405.1000).
4.1.2.2.40. 2-(1-Propoxy-1H-indol-3-yl)-N-(2-(trifluoromethyl)phenyl)acetamide (6q)
Four steps yield 4.2%. 1H NMR (400 MHz, acetone-d 6): δ 8.30 (s, 1H, CONH), 8.11 (d, J = 8.4 Hz, 1H, ArH), 7.61 (m, 4H, ArH), 7.46 (d, J = 8.4 Hz, 1H, ArH), 7.29 (t, J = 7.6 Hz, 1H, ArH), 7.24 (t, J = 7.6 Hz, 1H, ArH), 7.09 (t, J = 7.6 Hz, 1H, ArH), 4.26 (t, J = 6.8 Hz, 2H, CH2), 3.90 (s, 2H, CH2), 1.83 (m, 2H, CH2), 1.08 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (150 MHz, acetone-d 6): δ 170.2, 136.8, 133.9, 133.7, 126.8 (q, C-CF3), 126.2, 125.7, 125.6, 125.5, 124.6, 123.9, 123.4, 120.7, 119.8, 109.2, 105.2, 80.8, 34.3, 22.3, 10.5; HR-ESIMS m/z 377.1477 [M + H]+ (Calcd for C20H20N2O2 F3, 377.1471).
4.1.2.2.41. 2-(1-Methoxy-1H-indol-3-yl)-N-(pyridin-2-yl)acetamide (10a)
Four steps yield 10.3%. 1H NMR (300 MHz, acetone-d 6): δ 9.36 (s, 1H, CONH), 8.22 (m, 2H, ArH), 7.70 (m, 2H, ArH), 7.52 (s, 1H, ArH), 7.43 (d, J = 7.8 Hz, 1H, ArH), 7.22 (t, J = 7.5 Hz, 1H, ArH), 7.08 (t, J = 7.5 Hz, 1H, ArH), 7.01 (dd, J = 4.5, 7.2 Hz, 1H, ArH), 4.07 (s, 3H, OCH3), 3.93 (s, 2H, CH2); 13C NMR (75 MHz, acetone-d 6): δ 170.5, 153.1, 148.7, 138.7, 133.3, 124.8, 123.6, 123.3, 120.5, 120.2, 120.1, 114.1, 109.0, 106.1, 66.2, 34.5; HR-ESIMS m/z 282.1239 [M + H]+ (Calcd for C16H16N3O2, 282.1237).
4.1.2.2.42. 2-(1-Methoxy-1H-indol-3-yl)-N-(pyridin-3-yl)acetamide (10b)
Four steps yield 9.3%. 1H NMR (400 MHz, acetone-d 6): δ 9.42 (s, 1H, CONH), 8.73 (s, 1H, ArH), 8.24 (d, J = 4.4 Hz, 1H, ArH), 8.12 (dd, J = 1.6, 8.4 Hz, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.48 (s, 1H, ArH), 7.42 (d, J = 8.0 Hz, 1H, ArH), 7.24 (m, 2H, ArH), 7.07 (t, 7.6 Hz, 1H, ArH), 4.08 (s, 3H, OCH3), 3.84 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.5, 145.2, 141.9, 136.9, 133.3, 127.0, 124.8, 124.2, 123.5, 123.2, 120.5, 120.1, 109.0, 106.1, 66.2, 34.4; HR-ESIMS m/z 282.1242 [M + H]+ (Calcd for C16H16N3O2, 282.1237).
4.1.2.2.43. 2-(1-Methoxy-1H-indol-3-yl)-N-(pyridin-4-yl)acetamide (10c)
Four steps yield 10.0%. 1H NMR (400 MHz, acetone-d 6): δ 9.59 (s, 1H, CONH), 8.39 (d, J = 5.6 Hz, 2H, ArH), 7.60 (m, 3H, ArH), 7.47 (s, 1H, ArH), 7.42 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 4.09 (s, 3H, OCH3), 3.84 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 171.0, 151.2 (2C), 146.9, 133.3, 124.8, 123.5, 123.2, 120.5, 120.1, 114.1 (2C), 109.0, 105.8, 66.2, 34.7; HR-ESIMS m/z 282.1243 [M + H]+ (Calcd for C16H16N3O2, 282.1237).
4.1.2.2.44. N-(3-Chloropyridin-4-yl)-2-(1-methoxy-1H-indol-3-yl)acetamide (10d)
Four steps yield 15.8%. 1H NMR (400 MHz, acetone-d 6): δ 8.69 (s, 1H, CONH), 8.42 (s, 1H, ArH), 8.36 (m, 2H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.63 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 4.12 (s, 3H, OCH3), 4.01 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.9, 149.9, 149.8, 142.5, 133.3, 124.5, 123.9, 123.6, 120.9, 120.2, 119.9, 115.1, 109.2, 105.2, 66.4, 34.6; HR-ESIMS m/z 316.086 [M + H]+ (Calcd for C16H15N3O2Cl, 316.0847).
4.1.2.2.45. N-(3-Bromopyridin-4-yl)-2-(1-methoxy-1H-indol-3-yl)acetamide (10e)
Four steps yield 16.9%. 1H NMR (400 MHz, acetone-d 6): δ 8.51 (s, 1H, CONH), 8.38 (d, J = 5.6 Hz, 1H, ArH), 8.33 (d, J = 5.6 Hz, 1H, ArH), 7.65 (m, 2H, ArH), 7.48 (d, J = 8.0 Hz, 1H, ArH), 7.26 (t, J = 7.6 Hz, 1H, ArH), 7.11 (t, J = 7.6 Hz, 1H, ArH), 4.14 (s, 3H, OCH3), 3.99 (s, 2H, CH2); 13C NMR (100 MHz, acetone-d 6): δ 170.8, 152.5, 150.3, 143.5, 133.4, 124.5, 124.1, 123.7, 121.0, 119.9, 115.3, 110.9, 109.2, 104.9, 66.5, 34.7; HR-ESIMS m/z 360.0345 [M + H]+ (Calcd for C16H15N3O2Br, 360.0342).
4.1.2.2.46. N-(3-Chloropyridin-4-yl)-2-(1-propoxy-1H-indol-3-yl)acetamide (10f)
Four steps yield 21.6%. 1H NMR (400 MHz, acetone-d 6): δ 8.66 (s, 1H, CONH), 8.42 (s, 1H, ArH), 8.36 (m, 2H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.63 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.24 (t, J = 7.6 Hz, 1H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 4.26 (t, J = 6.8 Hz, 2H, CH2), 4.00 (s, 2H, CH2), 1.81 (m, 2H, CH2), 1.08 (t, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 170.9, 149.9, 149.8, 142.5, 133.8, 124.7, 124.5, 123.5, 120.8, 119.9, 115.0, 114.9, 109.3, 104.9, 80.8, 34.7, 22.3, 10.5; HR-ESIMS m/z 344.1166 [M + H]+ (Calcd for C18H19N3O2Cl, 344.1160).
4.1.2.2.47. N-(3-Fluoropyridin-4-yl)-2-(1-propoxy-1H-indol-3-yl)acetamide (10g)
Four steps yield 10.3%. 1H NMR (400 MHz, acetone-d 6): δ 9.29 (s, 1H, CONH), 8.36 (m, 2H, ArH), 8.28 (d, J = 6.4 Hz, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.52 (s, 1H, ArH), 7.43 (d, J = 7.6 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.07 (t, J = 7.6 Hz, 1H, ArH), 4.24 (t, J = 6.4 Hz, 2H, CH2), 3.98 (s, 2H, CH2), 1.79 (m, 2H, CH2), 1.07 (t, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 171.1, 147.45, 147.39, 138.0, 137.8, 133.8, 124.7, 124.3, 123.3, 120.5, 120.0, 115.6, 110.6, 109.2, 105.4, 80.6, 34.4, 22.3, 10.5; HR-ESIMS m/z 328.1458 [M + H]+ (Calcd for C18H18N3O2F, 328.1456).
4.1.2.2.48. 2-(1-Propoxy-1H-indol-3-yl)-N-(pyrimidin-4-yl)acetamide (10h)
Four steps yield 7.2%. 1H NMR (400 MHz, acetone-d 6): δ 9.73 (s, 1H, CONH), 8.75 (s, 1H, CONH), 8.59 (d, J = 5.6 Hz, 1H, ArH), 8.13 (d, J = 5.6 Hz, 1H, ArH), 7.67 (d, J = 8.0 Hz, 1H, ArH), 7.53 (s, 1H, ArH), 7.43 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.07 (t, J = 6.4 Hz, 1H, ArH), 4.23 (t, J = 6.4 Hz, 2H, CH2), 3.98 (s, 2H, CH2), 1.79 (m, 2H, CH2), 1.06 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 171.8, 159.2, 159.0, 158.6, 133.8, 124.7, 124.4, 123.2, 120.5, 120.1, 110.5, 109.2, 105.2, 80.6, 34.5, 22.3, 10.5; HR-ESIMS m/z 311.1513 [M + H]+ (Calcd for C17H19N4O2, 311.1503).
4.1.2.2.49. 2-(1-Butoxy-1H-indol-3-yl)-N-(3-chloropyridin-4-yl)acetamide (10i)
Four steps yield 20.5%. 1H NMR (400 MHz, acetone-d 6): δ 8.66 (s, 1H, CONH), 8.42 (s, 1H, ArH), 8.36 (m, 2H, ArH), 7.64 (m, 2H, ArH), 7.47 (d, J = 8.4 Hz, 1H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 4.31 (t, J = 7.2 Hz, 2H, CH2), 4.00 (s, 2H, CH2), 1.78 (m, 2H, CH2), 1.54 (m, 2H, CH2), 0.98 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 170.9, 149.9, 149.8, 142.5, 133.9, 124.7, 124.5, 123.6, 120.8, 119.9, 115.03, 114.96, 109.3, 104.9, 79.1, 34.7, 31.0, 19.7, 14.1; HR-ESIMS m/z 358.1321 [M + H]+ (Calcd for C19H21N3O2Cl, 358.1317).
4.1.2.2.50. N-(3-Chloropyridin-4-yl)-2-(1-(pentyloxy)-1H-indol-3-yl)acetamide (10j)
Four steps yield 20.2%. 1H NMR (400 MHz, acetone-d 6): δ 8.66 (s, 1H, CONH), 8.42 (s, 1H, ArH), 8.36 (m, 2H, ArH), 7.64 (m, 2H, ArH), 7.47 (d, J = 8.4 Hz, 1H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 4.31 (t, J = 7.2 Hz, 2H, CH2), 4.00 (s, 2H, CH2), 1.81 (m, 2H, CH2), 1.50 (m, 2H, CH2), 1.40 (m, 2H, CH2)0.93 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 170.9, 149.9, 149.8, 142.5, 133.9, 124.7, 124.5, 123.6, 120.8, 119.9, 115.0, 109.3, 104.9, 79.4, 34.7, 28.69, 28.68, 23.1, 14.2; HR-ESIMS m/z 372.1484 [M + H]+ (Calcd for C20H23N3O2Cl, 372.1473).
4.1.2.3. Synthesis of 14 and 15
To a cold solution of 11 in MeOH (0 °C) was added sodium tungstate dehydrate (0.3 equiv.) and dropwise hydrogen peroxide (30%, 10 equiv.), stirred at 10–15 °C for 1 h, then added CH2Cl2 and water and stirred for 10 min. After separation, washed with brine, and dried over anhydrous Na2SO4, the organic phase was evaporated to give a residue; then added DMF, K2CO3 (3 equiv.), and the alkyl halide (1.5 equiv.), stirred at room temperature for 2 h, and followed by addition of water and ethyl acetate then stirring for 10 min. The organic layer was separated, dried over anhydrous Na2SO4, and evaporated to give a residue. The residue was dissolved in diethyl ether, added oxalyl chloride (1.5 equiv.) dropwise at 0 °C, and stirred at room temperature for 2 h. After removal of the diethyl ether and re-dissolving in CH2Cl2, to the solution was added 4-amino-3-chloropyridine (1.2 equiv.) and triethylamine (2.0 equiv.) dropwise at 0 °C then stirred at room temperature for 3 h. Evaporation of the solvent gave a residue that was purified by silica gel column chromatography (petroleum ether/ethyl acetate 1:1) to furnish 14. To a solution of 14 (1 equiv.) in methanol was added sodium borohydride (3.0 equiv.), stirred for 1 h, then diluted with water and extracted with ethyl acetate. The ethyl acetate layer was washed with brine then evaporated to afford a residue, which was purified by silica gel column chromatography (petroleum ether/ethyl acetate 1:1) to obtain 15.
4.1.2.3.1. N-(3-Chloropyridin-4-yl)-2-oxo-2-(1-propoxy-1H-indol-3-yl)acetamide (14)
Three steps yield 45.7%. 1H NMR (400 MHz, acetone-d 6): δ 10.11 (s, 1H, CONH), 9.16 (s, 1H, ArH), 8.63 (s, 1H, ArH), 8.53 (d, J = 5.6 Hz, 1H, ArH), 8.48 (d, J = 5.6 Hz, 1H, ArH), 8.41 (d, J = 7.2 Hz, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.41 (m, 1H, ArH), 4.49 (t, J = 6.4 Hz, 2H, CH2), 1.90 (m, 2H, CH2), 1.13 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO‑d 6): δ 178.5, 160.9, 149.5, 149.1, 140.5, 135.7, 132.2, 124.8, 124.2, 123.1, 121.71, 121.66, 115.8, 109.7, 106.4, 81.2, 21.0, 10.0; HR-ESIMS m/z 358.0971 [M + H]+ (Calcd for C18H17N3O3Cl, 358.0953).
4.1.2.3.2. N-(3-Chloropyridin-4-yl)-2-hydroxy-2-(1-propoxy-1H-indol-3-yl)acetamide (15)
Yield 79%. 1H NMR (400 MHz, DMSO‑d 6): δ 9.93 (s, 1H, CONH), 8.65 (s, 1H, ArH), 8.44 (d, J = 5.2 Hz, 1H, ArH), 8.26 (d, J = 5.6 Hz, 1H, ArH), 7.71 (s, 1H, ArH), 7.67 (d, J = 8.0 Hz, 1H, ArH), 7.43 (d, J = 8.4 Hz, 1H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 6.98 (d, J = 3.6 Hz, 1H, OH), 5.48 (d, J = 4.0 Hz, 1H, CH), 4.19 (t, J = 6.8 Hz, 2H, CH2), 1.72 (m, 2H, CH2), 1.01 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO‑d 6): δ 171.8, 149.21, 149.16, 140.7, 132.4, 123.6, 122.4, 121.7, 119.9 (2C), 119.8, 114.1, 110.0, 108.4, 79.7, 68.1, 21.1, 10.1; HR-ESIMS m/z 360.1115 [M + H]+ (Calcd for C18H19N3O3Cl, 360.1109).
4.1.2.4. General procedure for the synthesis of 20
To a solution of 16 (1.0 mmol, 1 equiv.) and a derivative of pyridine formaldehyde (1.1 equiv.) in 2 mL of MeOH was added an aqueous solution of sodium hydroxide (4 M, 2 equiv.) at 0–5 °C in 10 min, and stirred at room temperature for 2 h. The formed precipitates were collected by filtration, washed with MeOH, and dried under vacuum to afford the intermediate 17 as a pale yellow solid. Subsequently 20 was synthesized from the intermediate 17 following the same reduction, N-oxidation, and alkylation as described for the synthesis of 5, 6, and 10.
4.1.2.4.1. 1-Propoxy-3-(pyridin-4-ylmethyl)-1H-indole (20a)
Four steps yield 33.8%. 1H NMR (400 MHz, acetone-d 6): δ 8.47 (d, J = 5.2 Hz, 2H, ArH), 7.45 (m, 2H, ArH), 7.37 (s, 1H, ArH), 7.31 (d, 2H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.02 (t, J = 7.6 Hz, 1H, ArH), 4.22 (t, J = 6.4 Hz, 2H, CH2), 4.11 (s, 2H, CH2), 1.79 (m, 2H, CH2), 1.07 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 151.7, 150.3 (2C), 134.1, 124.9 (2C), 124.4, 123.7, 123.2, 120.3, 119.9, 109.9, 109.2, 80.5, 31.1, 22.3, 10.5; HR-ESIMS m/z 267.1498 [M + H]+ (Calcd for C18H19N2O, 267.1492).
4.1.2.4.2. 3-[(3-Chloropyridin-4-yl)methyl]-1-propoxy-1H-indole (20b)
Four steps yield 29.3%. 1H NMR (400 MHz, acetone-d 6): δ 8.53 (s, 1H, ArH), 8.34 (d, J = 4.4 Hz, 1H, ArH), 7.49 (d, J = 7.6 Hz, 1H, ArH), 7.44 (d, J = 8.0 Hz, 1H, ArH), 7.37 (s, 1H, ArH), 7.21 (m, 2H, ArH), 7.04 (t, J = 7.6 Hz, 1H, ArH), 4.23 (m, 4H, CH2 × 2), 1.79 (m, 2H, CH2), 1.06 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, acetone-d 6): δ 149.8, 148.9 (2C), 148.2, 134.0, 126.0, 124.4, 124.2, 123.3, 120.5, 119.8, 109.3, 108.1, 80.6, 28.6, 22.3, 10.5; HR-ESIMS m/z 301.1116 [M + H]+ (Calcd for C17H18N2OCl, 301.1102).
4.1.2.4.3. 3-[(3,5-Dichloropyridin-4-yl)methyl]-1-propoxy-1H-indole (20c)
Four steps yield 23.7%. 1H NMR (400 MHz, acetone-d 6): δ 8.53 (s, 2H, ArH), 7.69 (d, J = 8.0 Hz, 1H, ArH), 7.41 (d, J = 8.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.09 (m, 2H, ArH), 4.36 (s, 2H, CH2), 4.15 (t, J = 6.4 Hz, 2H, CH2), 1.72 (m, 2H, CH2), 1.00 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (125 MHz, acetone-d 6): δ 148.6 (2C), 145.9, 133.9, 133.6 (2C), 124.3, 123.4, 123.3, 120.5, 119.8, 109.2, 107.0, 80.5, 27.2, 22.2, 10.5; HR-ESIMS m/z 335.0717 [M + H]+ (Calcd for C17H17N2OCl2, 335.0712).
4.2. Biology
4.2.1. Cells and plasmids
HEK 293T cells were from the China Infrastructure of Cell Line Resource (Beijing, China) and cultured in Dulbecco’s modified Eagle’s medium containing 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37 °C under 5% CO2. The pNL4-3.luc.R−E- plasmid was acquired from the National Institute of Health AIDS Research and Reference Reagent Program. Vesicular stomatitis virus glycoprotein (VSVG) plasmid was kindly provided by Dr. Lijun Rong (University of Illinois at Chicago). The pNL4-3.luc.R−E- plasmids with RT mutations (pNL4-3.luc.R−E- RT-mutant) were constructed as described previously [56].
4.2.2. Anti-HIV-1 activity assay by pseudoviruses
VSVG plasmid and env-deficient HIV vector containing luciferase reporter (pNL4-3.luc.R−E- or pNL4-3.luc.R−E- RT-mutant) were co-transfected into HEK 293T cells. 48 h post transfection, the supernatant was collected and filtered through 0.45 μm filters, the harvested VSVG/HIV-1 or VSVG/HIV-1RT-mutant virions were quantified by using an ELISA kit (ZeptoMetrix, Cat. 0801111). HEK 293T cells were seeded on 48-well plates at a density of 3 × 104 cells/well one day prior to infection . The tested compounds were added to wells 15 min ahead of VSVG/HIV-1 or VSVG/HIV-1RT-mutant infection (0.1 ng p24/well). The infected cells were lysed 48 h postinfection, and the luciferase activity was measured using a Sirius luminometer (Berthold Detection System) according to the manufacturer’s instructions. Infectivity (%) = luciferase activity tested compound/luciferase activity DMSO × 100 [56].
4.2.3. Cell viability assay
HEK 293T cells, 4 × 103 cells/well in a 96-well plate, were treated with the compounds at the indicated concentration for 48 h. The cell viability was measured by the CellTiter-Glo® Assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Cells treated with the same amount DMSO (1‰) served as the solvent control. The cell viability of the each tested compound was calculated as Cell viability (%) = RLUs tested compound/RLUs DMSO × 100% [74].
4.2.4. Time-of-drug addition (TOA) assay
HEK 293T cells were seeded in 48-well plates one day before infection. The tested compounds and reference drugs were added into the different wells at the time points of 0, 2, 4, 6, 8, 10, 12, 14, 16 h of VSVG/HIV-1 infection. The final concentrations for 6d, 10f, 10i and reference drugs AZT, EFV, RAL were 3 μM, 0.5 μM, 0.3 μM, 1 μM, 1 μM, and 1 μM respectively. The luciferase activity of cell lysate was measured 48 h postinfection [75].
4.2.5. RT RNA-Dependent DNA polymerase activity detection
HIV-1 reverse transcriptase RNA-dependent DNA polymerase activity was detected as described previously [59]. The tested compound was added into 60 μL reaction system containing 11.7 μg/mL poly(rA), 1.125 μg/mL oligo(dT)15, 2.8 μM dTTP, 800 nM Digoxigenin-11-dUTP, 40 nM Biotin-11-dUTP, 50 mM Tris-HCl (pH 7.8), 290 mM KCl, 30 mM MgCl2 and 10 mM DTT. The reaction was initiated by RT addition. Blank control was conducted without adding RT, while solvent control was to add DMSO instead of the tested compound. The mixture was incubated at 37 °C for 1 h followed by being transferred into a streptavidin-coated plate (Roche) for 1 h incubation at 37 °C. The supernatant was then discarded and the plate was washed with PBS. Anti-DIG-POD was added to the plate and incubated at 37 °C for 1 h. The plate was washed and TMB (3,3,5,5-tetramethylbenzidine) was added. After 15 min, 1 M H2SO4 was added to terminate the reaction, and the OD450 values were read by a multimode reader (Tecan). RNA-Dependent DNA polymerase activity = (A450 tested compound – A450 blank control)/(A450 solvent control – A450 blank control) × 100%.
4.2.6. Ribonuclease H activity detection
Detection of Ribonuclease H activity was performed as described previously [60]. Briefly, the 1 μL tested compound was added into 49 μL reaction system which containing 50 mM Tris-HCl (pH 8.0), 60 mM KCl, 10 mM MgCl2, and RT. Then 50 μL RNA/DNA substrate solution containing 50 mM Tris-HCl (pH 8.0), 60 mM KCl and 4.5 μM RNA/DNA substrate (TriLink Biotechnologies) were added into the reaction system to react for 30 min at room temperature. Blank control was conducted without adding RT, while solvent control was to add DMSO instead of the tested compound. The reaction was terminated by adding 50 μL 0.5 M EDTA. The relative fluorescence units (RFU) were detected by multimode reader (Tecan) (Ex = 490 nm, Em = 528 nm). Ribonuclease H activity = (RFU tested compound –RFU blank control)/(RFU solvent control –RFU blank control) × 100%.
4.2.7. Statistical analysis
Mean value, standard deviation (SD), half maximal effective concentration (EC50), half maximal inhibitory concentration (IC50) and 50% cytotoxic concentration (CC50) were calculated by GraphPad Prism software.
4.3. Molecular docking
The molecular docking was performed with the Discovery Studio Client v18.1.0.17334 software package (Accelrys, San Diego, USA). The complex structure was obtained from the Protein Data Bank (WT RT: 1TL1, Y181C RT: 1JLB, K103N–Y181C double-mutated RT: 5VQY, L100I–K103N double-mutated RT: 2ZE2). In the complex, water molecules and the original ligand were removed, and the binding cavity of active site was re-docked with the compound to be tested, both the structures of receptor and ligand for docking were energy optimized. The docking was carried out with the CDOCKER protocol that employs CHARMm. All calculations were performed on a DELL OptiPlex 7040 workstation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support of the National Natural Science Foundation of China (81630094 and 81473256), CAMS Innovation Fund for Medical Science of China (2017-I2M-3-010 and 2016-I2M-1-014), the Science and Technology Program of Beijing (Z151100000115008), and the Drug Innovation Major Project of China (2018ZX09711001-004-004, 2018ZX09711001-003-002, and 2015ZX09102-023) is acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmech.2020.112071.
Contributor Information
Ying Guo, Email: yingguo6@imm.ac.cn.
Jiangong Shi, Email: shijg@imm.ac.cn.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- Anti-DIG-POD
anti-digoxigenin peroxidase
- AZT
zidovudine
- CC
column chromatography
- CC50
50% cytotoxic concentration
- DMAP
4-dimethylaminopyridine
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- EC50
half maximal effective concentration
- EDCI
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- EDTA
ethylenediaminetetraacetic acid
- EFV
efavirenz
- FBS
fetal bovine serum
- HAART
highly active antiretroviral therapy
- HEK
human embryonic kidney
- HIV
human immunodeficiency virus
- 1H–1H COSY
1H–1H correlation spectroscopy
- HMBC
heteronuclear multiple bond correlation
- HPLC
high performance liquid chromatography
- HRMS
high resolution mass spectrum
- IN
integrase
- IC50
half maximal inhibitory concentration
- m-CPBA
3-chloroperbenzoic acid
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NVP
nevirapine
- OD450
optical density at 450 nm
- PBS
phosphate buffer saline
- PR
protease
- RAL
raltegravir
- RT
reverse transcriptase
- SAR
structure-activity relationship
- TOA
time-of-drug addition
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Douek D.C., Roederer M., Koup R.A. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2018. July 18). HIV/AIDS.https://www.who.int/en/news-room/fact-sheets/detail/hiv-aids Retrieved from. [Google Scholar]
- 3.United States Department of Health and HumanServices . 31 July 2019. Global Statistics." HIV.Gov.www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics [Google Scholar]
- 4.Zhang X. Anti-retroviral drugs: current state and development in the next decade. Acta Pharm. Sin. B. 2018;8:131–136. doi: 10.1016/j.apsb.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J.M., Ba M.Y., Yang Y., Yao C.S., Yu M., Shi J.G., Guo Y. Discovery of a semi-synthesized cyclolignan as a potent HIV-1 non-nucleoside reverse transcriptase inhibitor. J. Asian Nat. Prod. Res. 2017;21:76–85. doi: 10.1080/10286020.2017.1417266. [DOI] [PubMed] [Google Scholar]
- 6.Engelman A., Cherepanov P. The structural biology of HIV-1: mechanistic and therapeutic insights. Nat. Rev. Microbiol. 2012;10:279–290. doi: 10.1038/nrmicro2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta R.K., Gregson J., Parkin N., Haile-Selassie H., Tanuri A., Andrade Forero L., Kaleebu P., Watera C., Aghokeng A., Mutenda N., Dzangare J., Hone S., Hang Z.Z., Garcia J., Garcia Z., Marchorro P., Beteta E., Giron A., Hamers R., Inzaule S., Frenkel L.M., Chung M.H., de Oliveira T., Pillay D., Naidoo K., Kharsany A., Kugathasan R., Cutino T., Hunt G., Avila Rios S., Doherty M., Jordan M.R., Bertagnolio S. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect. Dis. 2018;18:346–355. doi: 10.1016/S1473-3099(17)30702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren J., Stammers D.K. Structural basis for drug resistance mechanisms for non-nucleoside inhibitors of HIV reverse transcriptase. Virus Res. 2008;134:157–170. doi: 10.1016/j.virusres.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Namasivayam V., Vanangamudi M., Kramer V.G., Kurup S., Zhan P., Liu X., Kongsted J., Byrareddy S.N. The journey of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) from lab to clinic. J. Med. Chem. 2019;62:4851–4883. doi: 10.1021/acs.jmedchem.8b00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill M.S.A., Hassan S.S., Ahemad N. Evolution of HIV-1 reverse transcriptase and integrase dual inhibitors: recent advances and developments. Eur. J. Med. Chem. 2019;179:423–448. doi: 10.1016/j.ejmech.2019.06.058. [DOI] [PubMed] [Google Scholar]
- 11.Tang J., Do H.T., Huber A.D., Casey M.C., Kirby K.A., Wilson D.J., Kankanala J., Parniak M.A., Sarafianos S.G., Wang Z. Pharmacophore-based design of novel 3-hydroxypyrimidine-2,4-dione subtypes as inhibitors of HIV reverse transcriptase-associated RNase H: tolerance of a nonflexible linker. Eur. J. Med. Chem. 2019;166:390–399. doi: 10.1016/j.ejmech.2019.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sang Y., Han S., Pannecouque C., De Clercq E., Zhuang C., Chen F. Ligand-based design of nondimethylphenyl-diarylpyrimidines with improved metabolic stability, safety, and oral pharmacokinetic profiles. J. Med. Chem. 2019;62:11430–11436. doi: 10.1021/acs.jmedchem.9b01446. [DOI] [PubMed] [Google Scholar]
- 13.Xiao T., Tang J.F., Meng G., Pannecouque C., Zhu Y.Y., Liu G.Y., Xu Z.Q., Wu F.S., Gu S.X., Chen F.E. Indazolyl-substituted piperidin-4-yl-aminopyrimidines as HIV-1 NNRTIs: design, synthesis and biological activities. Eur. J. Med. Chem. 2019:111864. doi: 10.1016/j.ejmech.2019.111864. [DOI] [PubMed] [Google Scholar]
- 14.Zhu M., Ma L., Wen J., Dong B., Wang Y., Wang Z., Zhou J., Zhang G., Wang J., Guo Y., Liang C., Cen S., Wang Y. Rational design and Structure-Activity relationship of coumarin derivatives effective on HIV-1 protease and partially on HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2019:111900. doi: 10.1016/j.ejmech.2019.111900. [DOI] [PubMed] [Google Scholar]
- 15.Han S., Sang Y., Wu Y., Tao Y., Pannecouque C., De Clercq E., Zhuang C., Chen F.E. Fragment hopping-based discovery of novel sulfinylacetamide-diarylpyrimidines (DAPYs) as HIV-1 nonnucleoside reverse transcriptase inhibitors. Eur. J. Med. Chem. 2020;185:111874. doi: 10.1016/j.ejmech.2019.111874. [DOI] [PubMed] [Google Scholar]
- 16.Tramontano E., Corona A., Menendez-Arias L. Ribonuclease H, an unexploited target for antiviral intervention against HIV and hepatitis B virus. Antivir. Res. 2019;171:104613. doi: 10.1016/j.antiviral.2019.104613. [DOI] [PubMed] [Google Scholar]
- 17.Jiangsu New Medical College . vol. 1. Shanghai Scientific and Technical Publishers; Shanghai: 1986. (Dictionary of Traditional Chinese Medicine). 126, 1250. [Google Scholar]
- 18.Hsuan S.L., Chang S.C., Wang S.Y., Liao T.L., Jong T.T., Chien M.S., Lee W.C., Chen S.S., Liao J.W. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J. Ethnopharmacol. 2009;123:61–67. doi: 10.1016/j.jep.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J., Jiang Z., Wang R., Zheng Y., Chen J., Zhang X., Ma Y. Isatisine A, a novel alkaloid with an unprecedented skeleton from Leaves of Isatis indigotica. Org. Lett. 2007;9:4127–4129. doi: 10.1021/ol701540y. [DOI] [PubMed] [Google Scholar]
- 20.Chen M., Gan L., Lin S., Wang X., Li L., Li Y., Zhu C., Wang Y., Jiang B., Jiang J., Yang Y., Shi J. Alkaloids from the root of Isatis indigotica. J. Nat. Prod. 2012;75:1167–1176. doi: 10.1021/np3002833. [DOI] [PubMed] [Google Scholar]
- 21.Chen M., Lin S., Li L., Zhu C., Wang X., Wang Y. Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1,2,4-thiadiazole rings from the root of Isatis indigotica. Org. Lett. 2012;14:5668–5671. doi: 10.1021/ol302660t. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Chen M., Wang F., Bu P., Lin S., Zhu C. Chemical constituents from root of Isatis indigotica, China. J. Chin. Mater. Med. 2013;38:1172–1182. [PubMed] [Google Scholar]
- 23.Liu Y.-F., Chen M.-H., Wang X.-L., Guo Q.-L., Zhu C.-G., Lin S., Xu C.-B., Jiang Y.-P., Li Y.-H., Jiang J.-D., Li Y., Shi J.-G. Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica. Chin. Chem. Lett. 2015;26:931–936. [Google Scholar]
- 24.Liu Y.F., Chen M.H., Guo Q.L., Lin S., Xu C.B., Jiang Y.P., Li Y.H., Jiang J.D., Shi J.G. Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J. Asian Nat. Prod. Res. 2015;17:689–704. doi: 10.1080/10286020.2015.1055729. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y.F., Chen M.H., Lin S., Li Y.H., Zhang D., Jiang J.D., Shi J.G. Indole alkaloid glucosides from the roots of Isatis indigotica. J. Asian Nat. Prod. Res. 2016;18:1–12. doi: 10.1080/10286020.2015.1117452. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y., Wang X., Chen M., Lin S., Li L., Shi J. Three pairs of alkaloid enantiomers from the root of Isatis indigotica. Acta Pharm. Sin. B. 2016;6:141–147. doi: 10.1016/j.apsb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M.-H., Lin S., Wang Y.-N., Zhu C.-G., Li Y.-H., Jiang J.-D., Shi J.-G. Antiviral stereoisomers of 3,5-bis(2-hydroxybut-3-en-1-yl)-1,2,4-thiadiazole from the roots of Isatis indigotica. Chin. Chem. Lett. 2016;27:643–648. [Google Scholar]
- 28.Liu Y., Chen M., Guo Q., Li Y., Jiang J., Shi J. Aromatic compounds from an aqueous extract of "ban lan gen" and their antiviral activities. Acta Pharm. Sin. B. 2017;7:179–184. doi: 10.1016/j.apsb.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng L., Guo Q., Liu Y., Chen M., Li Y., Jiang J., Shi J. Indole alkaloid sulfonic acids from an aqueous extract of Isatis indigotica roots and their antiviral activity. Acta Pharm. Sin. B. 2017;7:334–341. doi: 10.1016/j.apsb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng L.J., Guo Q.L., Xu C.B., Zhu C.G., Liu Y.F., Chen M.H., Lin S., Li Y.H., Jiang J.D., Shi J.G. Diglycosidic indole alkaloid derivatives from an aqueous extract of Isatis indigotica roots. J. Asian Nat. Prod. Res. 2017;19:529–540. doi: 10.1080/10286020.2017.1320547. [DOI] [PubMed] [Google Scholar]
- 31.Meng L., Guo Q., Liu Y., Shi J. 8,4’-Oxyneolignane glucosides from an aqueous extract of "ban lan gen" (Isatis indigotica root) and their absolute configurations. Acta Pharm. Sin. B. 2017;7:638–646. doi: 10.1016/j.apsb.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng L.-J., Guo Q.-L., Zhu C.-G., Xu C.-B., Shi J.-G. Isatindigodiphindoside, an alkaloid glycoside with a new diphenylpropylindole skeleton from the root of Isatis indigotica. Chin. Chem. Lett. 2018;29:119–122. [Google Scholar]
- 33.Meng L., Guo Q., Chen M., Jiang J., Li Y., Shi J. Isatindolignanoside A, a glucosidic indole-lignan conjugate from an aqueous extract of the Isatis indigotica roots. Chin. Chem. Lett. 2018;29:1257–1260. [Google Scholar]
- 34.Guo Q., Xu C., Chen M., Lin S., Li Y., Zhu C., Jiang J., Yang Y., Shi J. Sulfur-enriched alkaloids from the root of Isatis indigotica. Acta Pharm. Sin. B. 2018;8:933–943. doi: 10.1016/j.apsb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seyed M.A. A comprehensive review on Phyllanthus derived natural products as potential chemotherapeutic and immunomodulators for a wide range of human diseases. Biocatal. Agricult. Biotechnol. 2019;17:529–537. [Google Scholar]
- 36.Habibi P., Daniell H., Soccol C.R., Grossi-de-Sa M.F. The potential of plant systems to break the HIV-TB link. Plant Biotechnol. J. 2019;17:1868–1891. doi: 10.1111/pbi.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes-Silva C.C., Salatino A., Barbosa-Motta L.B., Negri G., Salatino M.L.F. Chemical characterization, antioxidant and anti-HIV activities of a Brazilian propolis from Ceará state. Rev. Bras. Farmacogn. 2019;29:309–318. [Google Scholar]
- 38.Chinsembu K.C. Chemical diversity and activity profiles of HIV-1 reverse transcriptase inhibitors from plants. Rev. Bras. Farmacogn. 2019;29:504–528. [Google Scholar]
- 39.H.D. Zhao, Y. Lu, M. Yan, C.H. Chen, S.L. Morris-Natschke, K.H. Lee, D.F. Chen, Rapid recognition and targeted isolation of anti-HIV daphnane diterpenes from Daphne genkwa guided by UPLC-MSn, J. Nat. Prod., DOI: 10.1021/acs.jnatprod.9b00993. [DOI] [PMC free article] [PubMed]
- 40.Sonar V.P., Corona A., Distinto S., Maccioni E., Meleddu R., Fois B., Floris C., Malpure N.V., Alcaro S., Tramontano E., Cottiglia F. Natural product-inspired esters and amides of ferulic and caffeic acid as dual inhibitors of HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2017;130:248–260. doi: 10.1016/j.ejmech.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 41.Pawar V., Lokwani D., Bhandari S., Mitra D., Sabde S., Bothara K., Madgulkar A. Design of potential reverse transcriptase inhibitor containing Isatin nucleus using molecular modeling studies. Bioorg. Med. Chem. 2010;18:3198–3211. doi: 10.1016/j.bmc.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Famiglini V., La Regina G., Coluccia A., Pelliccia S., Brancale A., Maga G., Crespan E., Badia R., Clotet B., Este J.A., Cirilli R., Novellino E., Silvestri R. New indolylarylsulfones as highly potent and broad spectrum HIV-1 non-nucleoside reverse transcriptase inhibitors. Eur. J. Med. Chem. 2014;80:101–111. doi: 10.1016/j.ejmech.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Corona A., Meleddu R., Esposito F., Distinto S., Bianco G., Masaoka T., Maccioni E., Menendez-Arias L., Alcaro S., Le Grice S.F., Tramontano E. Ribonuclease H/DNA polymerase HIV-1 reverse transcriptase dual inhibitor: mechanistic studies on the allosteric mode of action of isatin-based compound RMNC6. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Gao P., Zhan P., Liu X. Substituted indoles as HIV-1 non-nucleoside reverse transcriptase inhibitors: a patent evaluation (WO2015044928) Expert Opin. Ther. Pat. 2016;26:629–635. doi: 10.1517/13543776.2016.1135902. [DOI] [PubMed] [Google Scholar]
- 45.Dousson C., Alexandre F.R., Amador A., Bonaric S., Bot S., Caillet C., Convard T., da Costa D., Lioure M.P., Roland A., Rosinovsky E., Maldonado S., Parsy C., Trochet C., Storer R., Stewart A., Wang J., Mayes B.A., Musiu C., Poddesu B., Vargiu L., Liuzzi M., Moussa A., Jakubik J., Hubbard L., Seifer M., Standring D. Discovery of the Aryl-phospho-indole IDX899, a highly potent anti-HIV non-nucleoside reverse transcriptase inhibitor. J. Med. Chem. 2016;59:1891–1898. doi: 10.1021/acs.jmedchem.5b01430. [DOI] [PubMed] [Google Scholar]
- 46.Brigg S., Pribut N., Basson A.E., Avgenikos M., Venter R., Blackie M.A., van Otterlo W.A.L., Pelly S.C. Novel indole sulfides as potent HIV-1 NNRTIs. Bioorg. Med. Chem. Lett. 2016;26:1580–1584. doi: 10.1016/j.bmcl.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Devale T.L., Parikh J., Miniyar P., Sharma P., Shrivastava B., Murumkar P. Dihydropyrimidinone-isatin hybrids as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg. Chem. 2017;70:256–266. doi: 10.1016/j.bioorg.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Meleddu R., Distinto S., Corona A., Tramontano E., Bianco G., Melis C., Cottiglia F., Maccioni E. Isatin thiazoline hybrids as dual inhibitors of HIV-1 reverse transcriptase. J. Enzym. Inhib. Med. Chem. 2017;32:130–136. doi: 10.1080/14756366.2016.1238366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pu C., Luo R.H., Zhang M., Hou X., Yan G., Luo J., Zheng Y.T., Li R. Design, synthesis and biological evaluation of indole derivatives as Vif inhibitors. Bioorg. Med. Chem. Lett. 2017;27:4150–4155. doi: 10.1016/j.bmcl.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Famiglini V., La Regina G., Coluccia A., Masci D., Brancale A., Badia R., Riveira-Munoz E., Este J.A., Crespan E., Brambilla A., Maga G., Catalano M., Limatola C., Formica F.R., Cirilli R., Novellino E., Silvestri R. Chiral indolylarylsulfone non-nucleoside reverse transcriptase inhibitors as new potent and broad spectrum anti-HIV-1 Agents. J. Med. Chem. 2017;60:6528–6547. doi: 10.1021/acs.jmedchem.6b01906. [DOI] [PubMed] [Google Scholar]
- 51.Li X., Gao P., Huang B., Zhou Z., Yu Z., Yuan Z., Liu H., Pannecouque C., Daelemans D., De Clercq E., Zhan P., Liu X. Discovery of novel piperidine-substituted indolylarylsulfones as potent HIV NNRTIs via structure-guided scaffold morphing and fragment rearrangement. Eur. J. Med. Chem. 2017;126:190–201. doi: 10.1016/j.ejmech.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Zhao T., Meng Q., Kang D., Ji J., De Clercq E., Pannecouque C., Liu X., Zhan P. Discovery of novel indolylarylsulfones as potent HIV-1 NNRTIs via structure-guided scaffold morphing. Eur. J. Med. Chem. 2019;182:111619. doi: 10.1016/j.ejmech.2019.111619. [DOI] [PubMed] [Google Scholar]
- 53.Hdoufane I., Stoycheva J., Tadjer A., Villemin D., Najdoska-Bogdanov M., Bogdanov J., Cherqaoui D. QSAR and molecular docking studies of indole-based analogs as HIV-1 attachment inhibitors. J. Mol. Struct. 2019;1193:429–443. [Google Scholar]
- 54.Kumar S., Gupta S., Abadi L.F., Gaikwad S., Desai D., Bhutani K.K., Kulkarni S., Singh I.P. Synthesis and in-vitro anti-HIV-1 evaluation of novel pyrazolo[4,3-c]pyridin-4-one derivatives. Eur. J. Med. Chem. 2019;183:111714. doi: 10.1016/j.ejmech.2019.111714. [DOI] [PubMed] [Google Scholar]
- 55.Esposito F., Sechi M., Pala N., Sanna A., Koneru P.C., Kvaratskhelia M., Naesens L., Corona A., Grandi N., di Santo R., D’Amore V.M., Di Leva F.S., Novellino E., Cosconati S., Tramontano E. Discovery of dihydroxyindole-2-carboxylic acid derivatives as dual allosteric HIV-1 integrase and reverse transcriptase associated ribonuclease H inhibitors. Antivir. Res. 2019;174:104671. doi: 10.1016/j.antiviral.2019.104671. [DOI] [PubMed] [Google Scholar]
- 56.Cao Y., Li S., Chen H., Guo Y. Establishment of pharmacological evaluation system for non-nucleoside reverse transcriptase inhibitors resistant HIV-1. Acta Pharm. Sin. 2009;44:355–361. [PubMed] [Google Scholar]
- 57.Kursanov D.N., Parnes Z.N., Loim N.M. Applications of ionic hydrogenation to organic synthesis. Synthesis. 1974;9:633–651. [Google Scholar]
- 58.Somei M., Kawasaki T. A new and simple synthesis of 1-hydroxyindole derivatives. Heterocycles. 1989;29:1251–1254. [Google Scholar]
- 59.Ma X., Li L., Zhu T., Ba M., Li G., Gu Q., Guo Y., Li D. Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013;76:2298–2306. doi: 10.1021/np400683h. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y., Cao Y., Liu H., Yan H., Guo Y., Shizukaol F. A new structural type inhibitor of HIV-1 reverse transcriptase RNase H. Acta Pharm. Sin. 2012;47:1011–1016. [PubMed] [Google Scholar]
- 61.Tomakinian T., Kouklovsky C., Vincent G. Investigation of the synthesis of benzofuroindolines from N-Hydroxyindoles: an O-Arylation/[3,3]-sigmatropic rearrangement sequence. Synlett. 2015;26:1269–1275. [Google Scholar]
- 62.Meng J., Du D., Xiong G., Wang W., Wang Y., Koshima H., Matsuura T. A dual pathway in the solid-state photoreaction of nitrobenzaldehydes with indole. J. Heterocycl. Chem. 1994;31:121–124. [Google Scholar]
- 63.Hu M., Xu C., Yang C., Zuo H., Chen C., Zhang D., Shi G., Wang W., Shi J., Zhang T. Discovery and evaluation of ZT55, a novel highly-selective tyrosine kinase inhibitor of JAK2(V617F) against myeloproliferative neoplasms. J. Exp. Clin. Cancer Res. 2019;38:49. doi: 10.1186/s13046-019-1062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarafianos S.G., Marchand B., Das K., Himmel D.M., Parniak M.A., Hughes S.H., Arnold E. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 2009;385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Figiel M., Krepl M., Poznanski J., Golab A., Sponer J., Nowotny M. Coordination between the polymerase and RNase H activity of HIV-1 reverse transcriptase. Nucleic Acids Res. 2017;45:3341–3352. doi: 10.1093/nar/gkx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian L., Kim M.S., Li H., Wang J., Yang W. Structure of HIV-1 reverse transcriptase cleaving RNA in an RNA/DNA hybrid. Proc. Natl. Acad. Sci. U. S. A. 2018;115:507–512. doi: 10.1073/pnas.1719746115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanford University Non-nucleoside RT inhibitor (NNRTI)-resistance mutations, Stanford HIV Drug-Resistance Database. https://hivdb.stanford.edu/pages/3DStructures/rt.html#R
- 68.Hsiou Y., Ding J., Das K., Clark A.D., Jr., Boyer P.L., Lewi P., Janssen P.A., Kleim J.P., Rosner M., Hughes S.H., Arnold E. The Lys103Asn mutation of HIV-1 RT: a novel mechanism of drug resistance. J. Mol. Biol. 2001;309:437–445. doi: 10.1006/jmbi.2001.4648. [DOI] [PubMed] [Google Scholar]
- 69.Hopkins A.L., Ren J., Milton J., Hazen R.J., Chan J.H., Stuart D.I., Stammers D.K. Design of non-nucleoside inhibitors of HIV-1 reverse transcriptase with improved drug resistance properties. 1. J. Med. Chem. 2004;47:5912–5922. doi: 10.1021/jm040071z. [DOI] [PubMed] [Google Scholar]
- 70.Ren J., Nichols C., Bird L., Chamberlain P., Weaver K., Short S., Stuart D.I., Stammers D.K. Structural mechanisms of drug resistance for mutations at codons 181 and 188 in HIV-1 reverse transcriptase and the improved resilience of second generation non-nucleoside inhibitors. J. Mol. Biol. 2001;312:795–805. doi: 10.1006/jmbi.2001.4988. [DOI] [PubMed] [Google Scholar]
- 71.Chan A.H., Lee W.G., Spasov K.A., Cisneros J.A., Kudalkar S.N., Petrova Z.O., Buckingham A.B., Anderson K.S., Jorgensen W.L. Covalent inhibitors for eradication of drug-resistant HIV-1 reverse transcriptase: from design to protein crystallography. Proc. Natl. Acad. Sci. U. S. A. 2017;114:9725–9730. doi: 10.1073/pnas.1711463114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das K., Bauman J.D., Clark A.D., Jr., Frenkel Y.V., Lewi P.J., Shatkin A.J., Hughes S.H., Arnold E. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1466–1471. doi: 10.1073/pnas.0711209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh S.K., Nagarajan R. Total synthesis of cruciferane via epoxidation/tandem cyclization sequence. RSC Adv. 2014;4:63147–63149. [Google Scholar]
- 74.Zhang X., Yan F., Tang K., Chen Q., Guo J., Zhu W., He S., Banadyga L., Qiu X., Guo Y. Identification of a clinical compound losmapimod that blocks Lassa virus entry. Antivir. Res. 2019;167:68–77. doi: 10.1016/j.antiviral.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang D., Guo J., Zhang M., Liu X., Ba M., Tao X., Yu L., Guo Y., Dai J. Oxazole-containing diterpenoids from cell cultures of Salvia miltiorrhiza and their anti-HIV-1 activities. J. Nat. Prod. 2017;80:3241–3246. doi: 10.1021/acs.jnatprod.7b00659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.