Highlights
-
•
BEI-inactivated PRRSV candidate vaccine was developed using local Kazakh viral strains.
-
•
Immune response and clinical disease were compared with a commercial PRRSV vaccine.
-
•
Compared to commercial vaccine our vaccine induced better cross-protective response.
-
•
Use of a potent adjuvant and local PRRSV strains in the vaccine formulation is beneficial.
Keywords: PRRSV, Killed vaccines, Binary ethylenimine, Neutralizing antibodies, Polymeric adjuvant, Cross-protection
Abstract
The efficacy of a novel BEI-inactivated porcine reproductive and respiratory syndrome virus (PRRSV) candidate vaccine in pigs, developed at RIBSP Republic of Kazakhstan and delivered with an adjuvant Montanide™ Gel 01 ST (D/KV/ADJ) was compared with a commercial killed PRRSV vaccine (NVDC-JXA1, C/KV/ADJ) used widely in swine herds of the Republic of Kazakhstan. Clinical parameters (body temperature and respiratory disease scores), virological and immunological profiles [ELISA and virus neutralizing (VN) antibody titers], macroscopic lung lesions and viral load in the lungs (quantitative real-time PCR and cell culture assay) were assessed in vaccinated and both genotype 1 and 2 PRRSV challenged pigs. Our results showed that the commercial vaccine failed to protect pigs adequately against the clinical disease, viremia and lung lesions caused by the challenged field isolates, Kazakh strains of PRRSV type 1 and type 2 genotypes. In contrast, clinical protection, absence of viremia and lung lesions in D/KV/ADJ vaccinated pigs was associated with generation of VN antibodies in both homologous vaccine strain LKZ/2010 (PRRSV type 2) and a heterogeneous type 1 PRRSV strain (CM/08) challenged pigs. Thus, our data indicated the induction of cross-protective VN antibodies by D/KV/ADJ vaccine, and importantly demonstrated that an inactivated PRRSV vaccine could also induce cross-protective response across the viral genotype.
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is an economically devastating disease in pigs that causes an estimated direct loss of greater than $664 million annually to the US pork industry (Chand et al., 2012, Holtkamp and Kliebenstein, 2011). The causative agent, PRRS virus (PRRSV), belongs to the family Arteriviridae, order Nidovirales, and it causes respiratory problems in pigs of all ages and reproductive failure in sows, including still births and mummification, birth of weak piglets and high pre-weaning mortality (Kimman et al., 2009, Rossow et al., 1994). Genetic and antigenic analyses have revealed two distinct PRRSV genotypes, European (type 1) and North American (type 2). Marked genetic and antigenic differences of up to 40% are observed between the type 1 and type 2 PRRSV genotypes (Kim et al., 2007, Nelsen et al., 1999). In addition, within the genotypes up to 30% genetic variation for type I and 21% for type II PRRSV do exist (Kim et al., 2007, Zimmerman et al., 2012). This implies complexity of the PRRSV and difficulties to develop protective vaccines (Li et al., 2010).
Vaccination is the only viable strategy practiced to control the clinical PRRS disease and transmission in pigs. Two types of PRRSV vaccines are commercially available, modified live virus (MLV) vaccine and a killed virus (KV) vaccine (Charerntantanakul, 2009). PRRS MLV vaccine has been shown to provide protective efficacy against PRRSV infection in the field; however, it provides limited protection against heterologous viruses. Additionally, PRRS MLV vaccine has the intrinsic risk of reversion to a virulent strain (Kwang et al., 1999). Though PRRS KV vaccine is safe to use in pigs, it elicits insufficient immunity in pigs (Lee et al., 2014). Overall, commercially available PRRS KV vaccine does not induce strong required immune response, and thus failed to protect pigs against viremia to homologous as well as heterologous viral challenges (Nilubol et al., 2004, Scortti et al., 2007, Zuckermann et al., 2007).
Interestingly, experimental studies showed that it is possible to induce production of virus neutralizing (VN) antibody response in naïve pigs by immunization with inactivated PRRSV (Misinzo et al., 2006, Renukaradhya et al., 2015a). Passive transfer studies demonstrated that the PRRS VN antibody response is responsible for viral clearance from the blood and lungs, and reduce transplacental transmission of the virus, thus played a significant role in protective immune response against PRRS (Lopez and Osorio, 2004, Osorio et al., 2002). Recent research efforts aimed to improve protective immune response to PRRS KV have been focused on utilizing potent vaccine adjuvants, which help to reduce viremia, lung lesions and clinical disease in homologous and heterogeneous PRRSV challenged pigs. Several types of vaccine adjuvants have been investigated for their ability to potentiate the immune response to PRRS vaccines (Charerntantanakul, 2009, Li et al., 2013), for example polymeric adjuvant Montanide™ Gel (Deville et al., 2012, Parker et al., 2008, Tabynov et al., 2015). Previous work in our laboratory demonstrated that Montanide™ Gel 01 potentiates the immune response of a candidate inactivated PRRS vaccine (Tabynov, 2014), but at that time the efficacy was not compared with the commercial vaccine.
Therefore, we developed a binary ethylenimine (BEI)-inactivated experimental PRRSV vaccine containing the PRRSV strain, Arterivirus/LKZ/2010 (LKZ/2010) (type 2), and coadministered intramuscularly with Montanide™ Gel 01 ST adjuvant. The vaccine virus was isolated in the territory of the Republic of Kazakhstan in 2010. Further, to elucidate efficacy of our candidate KV PRRS vaccine, vaccinated piglets were challenged with the LKZ/2010 (parental type 2 vaccine strain) or Kostanay-CM/08 (type 1 heterogeneous strain) of PRRSV.
2. Materials and methods
2.1. Virus production
PRRSV strain LKZ/2010 (National Repository of Especially Dangerous Diseases of the Republic of Kazakhstan, registration number M-2-11/D) was propagated in MARC-145 cells to use in our experimental vaccine preparation. Virus infected cell culture supernatant at fifth passage was harvested after 96 h of infection and centrifuged at 3000 rpm for 20 min, and filtered through 0.45 μm filter. Virus titers were determined using a confluent monolayer of MARC-145 cells cultured for 48 h in 96-well tissue culture plates, and the viral titer was expressed in TCID50/ml (Reed and Muench, 1938).
2.2. Virus inactivation and analysis of its complete inactivation
Inactivation of PRRSV with BEI was performed as described (Bahnemann, 1990) with some modifications (Azanbekova et al., 2014). Briefly, BEI 0.1 M stock solution was prepared by cyclization of 2-bromoethylamine in 0.175 M NaOH for 1 h at 37 °C and stored at 4 °C. Virus was inactivated by incubation with 1 mM BEI for 30 h at 22 °C, and the unutilized BEI was neutralized by incubation with 0.2 mM sodium thiosulphate at 4 °C overnight.
To confirm complete inactivation, the inactivated virus suspension was inoculated onto MARC-145 cells cultured in 150 cm2 tissue culture flasks in 50 mL medium. The cells were cultivated for 3 week at 37 °C, during which time they were passaged three times and the absence of CPE was confirmed. Furthermore, 2 mL of inactivated PRRSV was injected into three 2-month-old pigs and serum samples collected every week for 7 weeks was assayed for viremia by real-time PCR as described in Section 2.12.
2.3. Preparation of vaccine formulation
After confirmation of viral inactivation, the viral antigen was mixed with a final concentration of 10% (w/w) Gel 01 ST polymeric adjuvant (Montanide™, SEPPIC, France) by manual shaking for 5 min.
2.4. Vaccines and challenge virus strains
The PRRSV vaccines used in this study were: (1) our candidate KV vaccine mixed with a polymeric adjuvant (D/KV/ADJ; LKZ/2010; RIBSP, Gvardeiskiy, Kazakhstan; Batch 0003); and (2) a commercial NVDC-JXA1 KV vaccine containing oil based adjuvant (C/KV/ADJ; Guangdong Dahuanong Animal Health Products Co., Ltd., Jinan, China; Batch 1305005). The challenge PRRSV strains include, PRRSV LKZ/2010 (type 2; isolated from Lugovskoi Konniy Zavod (LKZ) farm, Zhambylskaya, Oblast, Kazakhstan) (Orynbayev et al., 2010) and CM/08 (type 1; isolated from a private farm in Zatobol village, Kostanayskiy rayon, Kazakhstan) (Tabynov et al., 2013), were obtained from the Depository of Especially Dangerous Infectious Diseases, RIBSP. The challenge virus strains LKZ/2010 and CM/08 were propagated in MARC-145 cells, and confirmed as type 1 and type 2 viruses by EZ-PRRSV™ MPX 4.0 Real Time RT-PCR using Target-Specific Reagents kit for the Rapid Identification & Differentiation of North American and European PRRS Viral RNA (Tetracore, MD, USA).
2.5. Experimental design of animal studies
Fifty Large White breed pigs weighing ∼20 kg each (average 2 months-old) were purchased from a specific-pathogen-free herd with certified records showing free from PRRSV, porcine parvovirus, porcine respiratory coronavirus, Aujeszky’s disease, classical swine fever virus, transmissible gastroenteritis virus, swine influenza virus and Mycoplasma hyopneumoniae. The negative PRRSV status of the animals was confirmed by serology using a commercial ELISA kit after arrival of the animals to our facility. Pigs were randomly assigned into six treatment groups: Groups 1 and 2, mock-vaccinated negative control groups (received 2 mL final concentration of 10% Gel 01 adjuvant); Groups 3 and 4, vaccinated three times (0, 21, 35 days post-first vaccination [DPFV]) with a volume of 2 mL D/KV/ADJ containing Gel 01 adjuvant. Groups 5 and 6 were vaccinated twice (0 and 28 DPFV) with 2 mL of commercial C/KV/ADJ vaccine containing oil adjuvant as per the manufacturer’s recommendations. The virus titers present in the D/KV/ADJ and C/KV/ADJ vaccines before inactivation were 105.5 TCID50/mL and 106.0 TCID50/mL, respectively. At 49 DPFV, the negative control groups 1–2 (n = 5 pigs/group) and groups 3–6 (n = 10 pigs/group) were challenged intranasally (105.7 TCID50) and intramuscularly (105.7 TCID50) with LKZ/2010 or CM/08 PRRSV (Table 1 ). Serum samples were collected from pigs at 0, 7, 14, 21, 28, 35, 42, 49, 51, 53, 55, 57, 59, 61 and 63 DPFV and stored at −70 °C until used in assays. Pigs were euthanized at 63 DPFV using the mixture of ketamine and xylazine as previously described (Tabynov et al., 2014) and performed necropsy.
Table 1.
Design of the experimental PRRSV vaccine study.
| Group | No. of animals | PRRSV vaccine | Adjuvant | Days of vaccination | Challenge viral strain |
|---|---|---|---|---|---|
| 1 | 5 | Mock-DMEM | Montanide™ Gel 01 ST | 0, 21 and 35 | LKZ/2010 |
| 2 | 5 | Mock-DMEM | Montanide™ Gel 01 ST | 0, 21 and 35 | CM/08 |
| 3 | 10 | D/KV/ADJ | Montanide™ Gel 01 ST | 0, 21 and 35 | LKZ/2010 |
| 4 | 10 | D/KV/ADJ | Montanide™ Gel 01 ST | 0, 21 and 35 | CM/08 |
| 5 | 10 | C/KV/ADJ | Oil-based adjuvant | 0 and 28 | LKZ/2010 |
| 6 | 10 | C/KV/ADJ | Oil-based adjuvant | 0 and 28 | CM/08 |
2.6. Animals and bioethics
Pigs were housed in our BSL2 isolation animal facility at the Research Institute for Biological Safety Problems Science Committee, Ministry of Education and Science of the Republic of Kazakhstan (RIBSP). Animals had free access to filtered water and unmedicated sterilized feed. Pigs were initially kept for acclimation period for 7 days before started vaccination. This study was carried out in compliance with national and international laws and guidelines on animal handling. The animal use protocol was approved by the Committee on the Ethics of Animal Experiments at the RIBSP (Permit Number: 1210/147).
2.7. PRRS ELISA
Serum samples collected from pigs were aliquoted and stored at −70 °C until used in the assays. ELISA for PRRSV antibodies was performed using a commercial kit (BIONOTE, Inc., Hwaseong, Korea) according to the manufacturer’s instructions. The optical density was measured at 450 nm using the Multiskan plus microplate reader (Labsystems, Vantaa, Finland). The presence or absence of PRRSV antibodies was determined by calculating the sample to positive (S/P) ratio. Samples were considered positive for PRRSV antibodies if the S/P ratio was >0.4.
2.8. Determination of PRRSV neutralization (VN) titer
PRRSV VN antibody titers in serum samples collected at 0, 7, 14, 21, 28, 35, 42, 49, 51, 53, 55, 57, 59, 61 and 63 DPFV were analyzed by indirect immunofluorescence assay (IFA) as previously described (Christopher-Hennings et al., 2001). Briefly, samples were subjected to UV-treatment for 45 min to inactivate any PRRSV and subjected to heat inactivation at 56 °C for 30 min to inactivate the complement function. Two-fold dilutions of test samples prepared in serum free DMEM (100 μl/well) were incubated with 50 μl of PRRSV (LKZ/2010 or CM/08) 200 TCID50 per well for 2 h at 37 °C, subsequently 100 μl of the suspension was transferred into 96-well microtiter plate containing confluent monolayer of MARC-145 cells and incubated for 2 h at 37 °C. Further, 100 μl/well of DMEM containing 2% fetal bovine serum was added to each well and incubated for 48 h at 37 °C in a CO2 incubator. The contents of the wells were removed from the plate and the cell monolayer was fixed with 80% acetone in water for 15 min and the plates were allowed to dry in fumehood for 20 min. Cells were rehydrated with 100 μl/well of PBS for 3–5 min. CPE in both the plates meant for virus titration (see paragraph 2.11) and VN titer were examined after treatment with 50 μl/well of mouse anti-PPRSV nucleocapsid protein specific mAb (SDOW-17) (1:5000) followed by Alexa-488 conjugated anti-mouse IgG (H + L) secondary antibody (1:3000). After each treatment plates were incubated at 37 °C for 2 h, and washed 4 times in between the treatments and observed under an inverted fluorescent microscope after mounting the cell monolayer with glycerol-PBS in 6:4 ratio (50 μl/well). The VN antibody titer was determined to be the reciprocal dilution ratio of the sample at which >90% inhibition of PRRSV-induced immunofluorescence was observed.
2.9. Clinical and pathological examinations
Rectal temperature was measured daily from 0 to 14 days post-challenge (DPC) and the threshold for fever was defined as 40.0 °C. Respiratory disorders were scored as: 0 = normal; 1 = mild dyspnea and/or tachypnea if the animal was stressed by handling for 45 s; 2 = mild dyspnea and/or tachypnea at rest; 3 = moderate dyspnea and/or tachypnea when stressed; 4 = moderate dyspnea and/or tachypnea at rest; 5 = severe dyspnea and/or tachypnea when stressed; 6 = severe tachypnea and/or dyspnea at rest. During necropsy the lungs were excised and macroscopic lung lesions were scored to estimate the percentage of the lungs affected by pneumonia as described (Halbur et al., 1995).
2.10. Preparation of lung homogenates
One gram of lung tissue was collected from each pig into a 15 ml conical tube containing 5 ml of DMEM, minced, and homogenized using the IKA T 25 ULTRA TURRAX High Speed Homogenizer (Cole-Parmer, IL, USA) for one min on ice. The clarified supernatant was collected by centrifugation at 400g for 5 min, aliquoted and stored at −70 °C until assayed.
2.11. Virus titration
MARC-145 cells were seeded onto 96-well Plates 24 h before infection. PRRSV supernatants were serially 10-fold diluted (from 10−1 to 10−9) and 200 μL of each dilution were plated in six replicate wells. Cell culture media without PRRSV was used as a control. Cells were incubated at 37 °C for 72 h in a 5% CO2 incubator, fixed and immunostained as described above (2.8. PRRSV VN titer assay). The titers were calculated as described previously (Reed and Muench, 1938) and expressed as TCID50/mL.
2.12. Quantitation of PRRSV by real-time PCR
PRRSV RNA was extracted from serum samples and lung homogenate using MagMax™-96 virus isolation kit (Ambion/Applied Biosystems, Carlsbad, California, USA) as per the manufacturer's instructions. Real-time RT-PCR was performed using the EZ-PRRSV™ MPX 4.0 assay (Tetracore®; Rockville, MD, USA), which covers two target regions of PRRSV type 1 and type 2 genes using specific primers and probes. In the assay, 6-FAM (6-carboxyfluorescein) was used as a reporter dye for detection of type 1 and type 2 PRRSV; and the alternative reporter dye CY5 was used for detection of extraction/inhibition control. The reaction volume per well included 17.25 μl of EZ-PRRSV™ MPX 4.0 Reagent (includes buffer, primer and probes), 0.75 μl of Enzyme Blend and 7 μl of the extracted serum sample. Each PCR run included two positive controls for type 1 and type 2 viruses and one negative amplification control. Negative and positive controls per well contained 17.25 μl of EZ-PRRSV™ MPX 4.0 Reagent, 0.75 μl of Enzyme Blend, 0.25 μl of inhibition control and 7 μl of the positive (3.5 μl of type 1 and 2 PRRSV) or negative control (1 × TE buffer). Plates were briefly mixed by shaking on a vortex mixer (10 s), centrifuged, and loaded into the thermal cycler (7500 Fast Real-Time PCR System; Applied Biosystems®, Foster City, CA, USA). The thermal cycling conditions were as follows: one cycle at 48 °C for 15 min, one cycle at 95 °C for 2 min, 45 cycles at 95 °C for 5 s and 60 °C for 40 s. Samples with Ct values < 38 for either genotype PRRSV were considered positive.
Quantification of samples was expressed in terms of the number of RNA copies per ml for serum and copies per gram of lung tissues. These estimates were based on linear extrapolation of the cycle threshold values against a standard curve generated by serial dilutions of known amounts of in vitro transcript RNA product (1 × 101 to 1 × 108 copies per μl).
2.13. Statistical analysis
All data were expressed as the mean ± standard error mean (SEM) value of 5–10 pigs in each group. The differences in body temperatures, lung pathology scores, humoral responses and viremia between groups were assessed by one-way ANOVA followed by Tukey’s multiple comparisons test. P-values < 0.05 were considered significant. All statistical analysis was performed using Graphpad Prism Software, version 6.0 (Graphpad Software Inc., San Diego, CA, USA).
3. Results
3.1. Clinical signs after vaccination and challenge
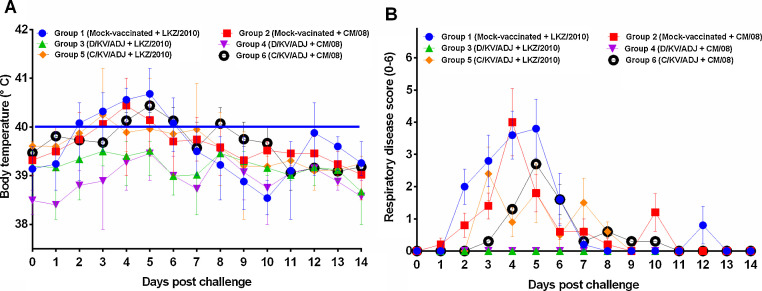
All animals remained in good health after vaccination with D/KV/ADJ or C/KV/ADJ vaccine and prior to viral challenge. Any local or systemic vaccine side effects were absent pre- and post-challenge and none of the experimental pigs died during the entire study period. The body temperature of the animals in every group fluctuated within the normal physiological range after vaccination, but not statistically significant (data not shown). After pigs were challenged with LKZ/2010 or CM/08, the body temperature in the D/KV/ADJ-vaccinated pig groups remained within the normal range (38.0–39.8 °C) during the entire study period (14 days). While the body temperature of the animals in the mock-vaccinated control and C/KV/ADJ-vaccinated pig groups were increased > 40.0 °C on days 2–6 and days 3–6 post-challenge, respectively (Fig. 1 A). There were significant differences in mean body temperature between the mock-vaccinated, C/KV/ADJ-vaccinated and D/KV/ADJ-vaccinated pigs following the viral challenge. The magnitude of increase in body temperature was correlated with the severity of clinical disease, i.e., lower the body temperature lower the overall severity of clinical disease. Severe respiratory disorders were observed after challenge with CM/08 and LKZ/2010 at 4 and 5 DPC, respectively, with significantly higher respiratory disease scores in the mock-vaccinated and C/KV/ADJ-vaccinated animals than the D/KV/ADJ-vaccinated pigs (Fig. 1B).
Fig. 1.
Body temperature (A) and respiratory disease scores (B) in pigs post-challenge. The body temperature ≥40 °C was considered as fever (solid blue line). Severity of respiratory disease was scored as described in Methods. Data shown are mean ± SEM of 5 or 10 pigs in each group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. PRRSV-specific antibody levels after vaccination and challenge
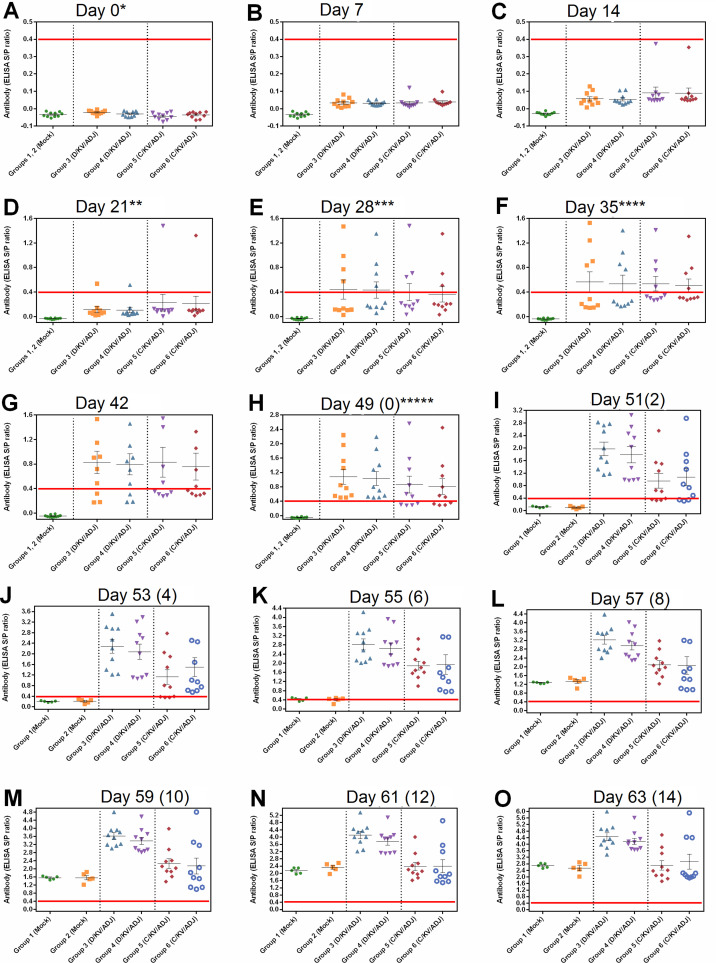
The BIONOTE ELISA demonstrated that all pigs were seronegative for PRRSV at DPFV and remained low until 21 DPFV (Fig. 2 A–C). The animals in the D/KV/ADJ- and C/KV/ADJ-vaccinated groups became PRRSV seropositive as follows: at DPFV 21 (10% and 0%), 28 (40% and 30%), 35 (40%), 42 (60% and 40%) and 49 (100% and 60%), respectively (Fig. 2D–H). This result indicated that the D/KV/ADJ vaccine induced greater PRRSV specific antibody production than the commercial C/KV/ADJ-vaccine in pigs.
Fig. 2.
Detection of PRRSV-specific antibodies in serum by ELISA. Positive responses were classified as the S/P ratio of ≥0.4 (horizontal red line). Serum samples were collected from the pigs at DPFV 0 (A), 7 (B), 14 (C), 21 (D), 28 (E), 35 (F), 42 (G), 49 (H) 51 (I), 53 (J), 55 (K), 57 (L), 59 (M), 61 (N) and 63 (O). *First vaccination; **second vaccination for groups 3 and 4; ***second vaccination for groups 5 and 6; ****third vaccination for groups 3 and 4; *****challenge. Data are mean ± SEM titer of 5 or 10 pigs in each group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
All the C/KV/ADJ-vaccinated pigs (100%) became seropositive at DPC 6 (Fig. 2I–K). At DPC 2 and 6, all the C/KV/ADJ vaccinated groups showed significantly higher S/P ratios compared to the control groups (P < 0.05). At DPC 8 and 14, the S/P ratios of the D/KV/ADJ-vaccinated groups were significantly higher than the C/KV/ADJ-vaccinated groups (P < 0.05; Fig. 2L–O). There was no significant difference between the S/P ratios of the C/KV/ADJ-vaccinated and mock-vaccinated pig groups at DPC 10 and 14 (Fig. 2M–O). Additionally, the antibody titers of the control groups 1 and 2 remained negative (Fig. 2A–O).
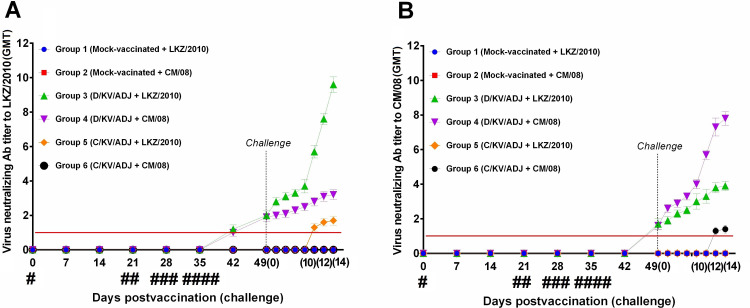
3.3. PRRSV-specific VN antibody levels after vaccination and challenge
Serum PRRS VN antibody titers in pigs were analyzed by the standard serum neutralization assay, and our results showed neither the D/KV/ADJ nor C/KV/ADJ vaccine induced VN antibodies to LKZ/2010 (type 2) or CM/08 (type 1) after the first or second vaccination. In the D/KV/ADJ-vaccinated pigs, VN titers against LKZ/2010 were detected at DPFV 42 (Fig. 3 A). The VN titers in the D/KV/ADJ-vaccinated groups were significantly higher compared to the C/KV/ADJ-vaccinated pigs at DPC 10 and 14 (P < 0.05 to P < 0.0001) (Fig. 3A). A rapid VN antibody response against LKZ/2010 virus was observed in the D/KV/ADJ vaccinated pig group 3 indicating that the vaccine induced better protective memory immune response. To assess broad VN antibody activity, we used heterologous PRRSV strain (type 1) CM/08 in VN assay and detected VN titers at DPFV 42 in pig groups 3 and 4 vaccinated with D/KV/ADJ (Fig. 3B). The VN titers of these animals were significantly higher than those of C/KV/ADJ-vaccinated pigs (group 6) at DPC 12–14 (P < 0.05 to P < 0.001) (Fig. 3B). The VN titers against the challenge virus strain of the pig group 4 vaccinated with D/KV/ADJ and challenged with CM/08 strain were similar to those of group 3 animals challenged with the homologous virus strain LKZ/2010. This indicates that our experimental pigs received PRRSV vaccination (strain LKZ/2010) followed by a heterologous CM/08 strain challenge generated antibodies with a broader neutralizing spectrum.
Fig. 3.
Serum virus neutralizing antibody titers post-vaccination and challenge. # = first vaccination; ## = second vaccination for groups 1–4; ### = second vaccination for groups 5 and 6; #### = third vaccination for groups 1–4. Serum samples were titrated on MARC-145 cells and the levels of PRRSV neutralizing antibodies to LKZ/2010 (A) and CM/08 (B) were determined as the reciprocal of the highest dilution that inhibited virus induced immunofluorescence (CPE). The solid red line indicates the detection limit for the VN assay. Data are geometric mean ± SEM of 5 or 10 pigs in each group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Pathological analysis and viremia
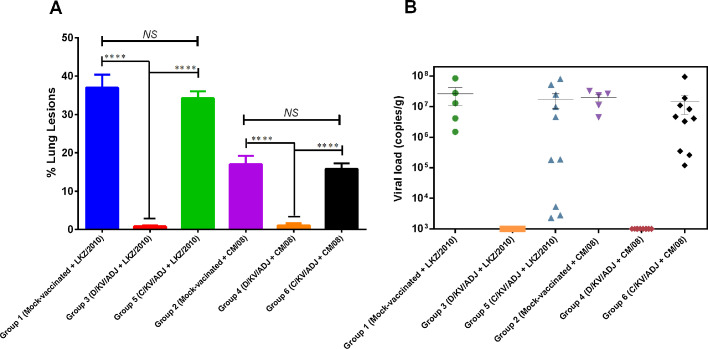
Significant difference in the macroscopic lung lesions scores were observed between pig groups 4 and 6 during necropsy at DPC 14. In a similar trend to the body temperature, less clinically-affected D/KV/ADJ-vaccinated pigs had significantly fewer lung lesions scores (P < 0.0001) compared to severely clinically-affected mock-vaccinated and C/KV/ADJ-vaccinated pigs (Fig. 4 A). All the mock-vaccinated and C/KV/ADJ-vaccinated pigs challenged with LKZ/2010 or CM/08 PRRSV had gross lung lesions in the cranial, middle and accessory lobes, which resulted in failure of the lungs to collapse during necropsy and lung parenchyma remained firm and rubbery. No obvious lung lesions were observed in D/KV/ADJ-vaccinated pig groups, indicating that this vaccine-adjuvant formulation enabled pigs to tolerate challenge with type 1 and type 2 PRRSV.
Fig. 4.
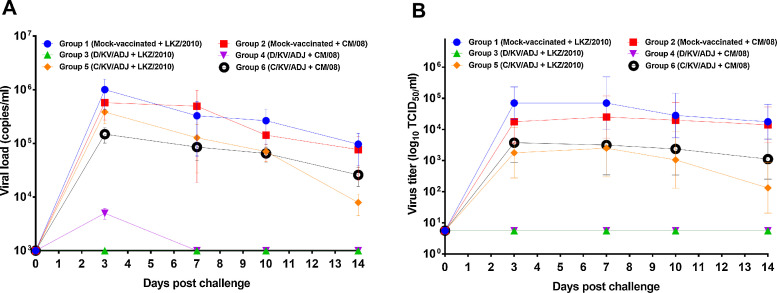
Macroscopic lung lesions (A) and quantification of PRRSV load by qRT-PCR (B). Lungs of pigs vaccinated and challenged with the PRRSV strains LKZ/2010 or CM/08 were collected at necropsy (DPC 14/63 DPFA). The viral RNA copy numbers in the lung homogenates were quantified by qRT-PCR and its limit of detection was 103 RNA copies/ml. Data is shown as mean ± SEM viral RNA copy numbers of 5 or 10 pigs in each group; ****P < 0.0001; analyzed by two-way ANOVA followed by Tukey’s multiple comparisons test.
Further, PRRSV RNA copy numbers in the lungs at necropsy (14 DPC) was measured using the commercial quantitative PCR (qPCR) kit. In pig groups that were immunized with D/KV/ADJ, PRRSV RNA copies were undetectable in 10/10 animals (< 103 RNA copies per gram of lung tissue). The viral loads of the mock-vaccinated and C/KV/ADJ-vaccinated pig groups were up to three logs (103) above PRRSV threshold detection limits; however, the viral loads of these groups were not significantly different (Fig. 4B). All the serum samples collected at DPFV 7, 14, 21, 28, 35, 42 and 49 were negative for PRRSV type 2 RNA copies (data not shown).
Viremia after challenge was assessed by both qPCR and viral titers on Marc-145 cells (Fig. 5 ). PRRSV RNA could not be detected in the serum samples obtained from the pigs vaccinated with D/KV/ADJ at any time point throughout the period post-challenge, except in group 4 pigs observed 4.99 × 103 copies at DPC 3 following challenge with the type 1 viral strain CM/08. Contrastingly, serum collected from pigs vaccinated with mock and C/KV/ADJ had viremia with the viral copy numbers from 1.48 × 105 to 1.00 × 106 copies (Fig. 5A).
Fig. 5.
PRRS viremia levels were measured by qPCR (A) and by assessing viral titers on Marc-145 cells (B). Data in (A) indicates the mean PRRSV ± SEM RNA copy numbers of 5 or 10 pigs in each group, and data in (B) were expressed as the geometric mean ± SEM log10TCID50/ml of 5 or 10 pigs in each group. Serum samples of pigs were collected up to DPC 14 (63 DPFA) challenged with PRRSV (LKZ/2010 or CM/08). The limit of detection by the assay kit was 103 RNA copies per ml. The limit of detection for the virus titration assay was 100.75 TCID50/ml.
The serum samples were also analyzed of virus titration using the Marc-145 cells (Fig. 5B). PRRSV was not detected in the serum of pig groups 3 and 4 collected at DPFV 7, 14, 21, 28, 35, 42 and 49, confirming that the virus was properly inactivated (data not shown). The mock-vaccinated pig groups challenged with LKZ/2010 or CM/08 exhibited the highest mean viral titer of 4.8 and 4.4 log10 TCID50/ml at 7 DPC in serum samples, respectively. All the animals vaccinated with C/KV/ADJ remained viremic until DPC 14. In contrast, D/KV/ADJ vaccinated pigs did not show viremia at all the DPCs by both RT-PCR and viral isolation methods (Fig. 5).
4. Discussion
Inactivated (killed) and MLV PRRSV vaccines are commercially available and licensed for use in many countries to control reproductive and respiratory forms of PRRS (Murtaugh and Genzow, 2011). However, each vaccine type possesses its own strengths and limitations. Due to safety issues inactivated vaccines are preferred over attenuated vaccines; however, efficacy of current inactivated PRRSV vaccines is questionable (Renukaradhya et al., 2015a, Scortti et al., 2007, Vanhee et al., 2009, Zuckermann et al., 2007). Inactivated PRRSV vaccine under field situations showed to elicit protective immune response under certain conditions, which was depending on the strain of infecting field virus. By employing a controlled inactivation procedure and using a suitable adjuvant, an inactivated PRRSV vaccine could be developed that systematically induces PRRSV specific VN antibody response after two vaccinations in naïve piglets (Nilubol et al., 2004). Serum VN antibodies have been identified as a key component of protective immunity against PRRSV infection (Osorio et al., 2002). Researchers have previously showed that an inactivated vaccine could induce VN antibodies resulting in strong to partial virological protection in challenged pigs (Misinzo et al., 2006, Renukaradhya et al., 2015a). Experiments of passive transfer of VN antibodies in pigs prior to PRRSV infection showed VN antibodies could fully protect pigs against viremia and reproductive failure (Lopez and Osorio, 2004, Osorio et al., 2002). However, others observed only low to moderate degree of protection against heterologous strains of virus when pigs were immunized with inactivated PRRSV vaccines (Labarque et al., 2004, Lager et al., 1999). But intranasal delivery of poly(lactic-co-glycolic) acid nanoparticle-entrapped UV-killed PRRSV vaccine adjuvanted with soluble Mycobacterium tuberculosis derived whole-cell lysate showed the induction of cross-protective immune response in pigs (Binjawadagi et al., 2014a, Binjawadagi et al., 2014b).
At present, it is generally accepted that there is a need for new and safe vaccines that protect against homologous and heterologous/heterogeneous PRRSV infections. In this study, our objective was to assess the efficacy of a candidate BEI-inactivated PRRSV vaccine (D/KV/ADJ), which contains a recently isolated PRRSV field strain (LKZ/2010) in Kazakhstan, against a homologous (type 2) and heterogeneous (type 1) viral challenges. A commercial killed PRRSV vaccine (C/KV/ADJ, strain NVDC-JXA1, type 2) was selected as a reference vaccine, which has been in use for immunization of pigs against PRRS in most of the pig farms in Kazakhstan. The efficacy of our vaccine candidate was assessed by evaluating its ability to reduce viremia and prevent clinical disease, including lung lesions in vaccinated pigs challenged with a homologous or heterogeneous PRRSV.
ELISA antibody analysis demonstrated that the commercial vaccine induced PRRSV-specific antibodies; however, only 60% of vaccinated pigs became seropositive prior to challenge. After challenge by day 6 all the C/KV/ADJ vaccinated pigs became seropositive. However, we did not observe VN titers to LKZ/2010 or CM/08 in the serum pre-challenge, and only a slightly elevated neutralizing antibody response was detected post-challenge in C/KV/ADJ-vaccinated pigs. These results are consistent with other published studies (Scortti et al., 2007, Zuckermann et al., 2007), wherein vaccination with inactivated PRRSV candidate vaccines slightly improve VN antibody response post-challenge. Moreover, comparable to mock-vaccinated pigs, C/KV/ADJ vaccinated animals became ill post-challenge and manifested characteristic clinical signs of PRRS (severe respiratory disorders with high scores), with simultaneous detection of viremia. Noticeable macroscopic lung lesions were observed at necropsy in all the mock- and C/KV/ADJ-vaccinated pigs challenged with LKZ/2010 (type 2) or CM/08 (type 1) virus, and detected high challenged viral RNA copy numbers in the lungs. Thus, we conclude that the commercial C/KV/ADJ vaccination in pigs does not protect against clinical disease, viremia and lung lesions against field variants of Kazakh strains of PRRSV. These results are in line with earlier studies, such as vaccination against PRRS in the field provides variable degrees of protection, and reports of clinical outbreaks of PRRS in vaccinated pigs have led to doubts about efficacy of current commercial PRRSV vaccines (Geldhof et al., 2012, Thanawongnuwech and Suradhat, 2010).
In contrast, ELISA results demonstrated that the D/KV/ADJ-vaccinated pigs produced PRRSV-specific antibodies more rapidly than the C/KV/ADJ and were 100% seropositive. After the third immunization, VN antibodies were detected in all the animals vaccinated with D/KV/ADJ prior to challenge, albeit at low levels. Interestingly, the VN titer did not reduce after challenge in any pig vaccinated with D/KV/ADJ, and the animals had consistently high VN titers against both homologous and heterogeneous PRRSV. This data is in agreement with an earlier study, which showed that high VN titers are required at the time of challenge to offer full protection against high dose of virus used to infect animals (Geldhof et al., 2012). The detectable levels of replicating PRRSV was absent post-challenge in D/KV/ADJ vaccinated pigs, which could be attributed to early appearance (pre-challenge) of VN antibodies targeting the putative neutralizing epitopes on the PRRSV GP5 and M proteins (Yang et al., 2000), but it needs further investigation. Inactivated vaccines predominantly elicit Th2 response (Spellberg and Edwards, 2001), but we cannot exclude the possibility of Th1-Th2-balanced response in D/KV/ADJ-vaccinated pigs, which also requires further research.
In the LKZ/2010 (type 2) and CM/08 (type 1) PRRSV strains challenged pigs vaccinated with D/KV/ADJ, we observed a strong association between the induction of virus-specific neutralizing antibody response and the absence of viremia, lung lesions and clinical disease; indicating that VN antibodies may have contributed significantly to cross-protection. Similar results were also observed in our previous study with this candidate vaccine, but challenged with a heterologous NADC-8 (type 2) strain of virus (Tabynov, 2014). These findings demonstrated that our candidate killed PRRSV vaccine has a safety profile, and it is efficacious when administered three times at days 0, 21 and 35 to Large-White breed of pigs aged 2 months.
The results of this study is interesting as the candidate killed PRRSV vaccine provided strong heterogeneous protection, because even modified live PRRSV vaccines have been shown ineffective against inter-genotypic virus and provided incomplete protection against reinfections and heterologous viruses (Botner et al., 1997, Kimman et al., 2009, Martelli et al., 2009, Renukaradhya et al., 2015a, Renukaradhya et al., 2015b). The reasons beyond better protective response by our candidate vaccine could be attributed to vaccination of pigs three times with Montanide™ SEPPIC adjuvant compared to others, wherein in many vaccine trials killed or subunit PRRSV vaccines were vaccinated twice using relatively weaker adjuvant than Montanide adjuvant (Renukaradhya et al., 2015a). Furthermore, at this moment due to lack of genome sequence data of both the vaccine and challenge viruses, it is difficult to draw any meaningful conclusions on this interesting result.
In this study, although our candidate BEI-inactivated PRRSV vaccine adjuvanted with Montanide™ Gel 01 ST provided protection against homologous (type 2) and heterogeneous (type 1) strains of virus, this does not confirm that this vaccine formulation would cross-protect against other heterogeneous and heterologous field strains of viruses; which needs further vaccine trials. Due to lack of sequence data of vaccine and challenge viruses used in this study, it is likely that though the commercial vaccine virus and the challenge virus strain are type 2 viruses, the level of genetic variability is not known. However, to our knowledge, this is the first report demonstrating that an inactivated PRRSV vaccine could also provide cross-protective response against a different viral genotype in pigs, which offers new perspectives for the development of effective and safe PRRSV vaccines.
Conflicts of interest
The authors declare no potential or actual conflict of interest.
Acknowledgments
This work was financially supported by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan under the following projects: 1833-GF “Development of manufacturing technology inactivated vaccine against porcine reproductive and respiratory syndrome” for 2012–2014; 1072/GF4 “Development of manufacturing technology of the bivalent inactivated vaccine against porcine reproductive and respiratory syndrome virus of European and North American genotypes” for 2015–2017. We thank Kulyaisan Sultankulova and Vitaliy Strochkov (Department of Molecular Biology and Genetic Engineering, RIBSP) for their help in RT-PCR analysis. In addition, we acknowledge the staff of the Laboratory Collection of Microorganisms of the RIBSP for excellent animal care assistance. Kairat Tabynov received a dissertation fellowship from Kazakh National Agrarian University (Republic of Kazakhstan) and the Norman E. Borlaug International Agricultural Science and Technology Fellowship Program 2015 of the U.S. Department of Agriculture.
References
- Azanbekova М.А., Mambetaliyev M., Azhibayev A.Z., Tabynov K.K., Khussainov D.M. Selection of effective activation agents for porcine reproductive and respiratory syndrome virus. Veterinary. 2014;2:50–55. (in Russian) [Google Scholar]
- Bahnemann H.G. Inactivation of viral antigens for vaccine preparation with particular reference to the application of binary ethylenimine. Vaccine. 1990;8:299–303. doi: 10.1016/0264-410X(90)90083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Torrelles J.B., Renukaradhya G.J. An innovative approach to induce cross-protective immunity against porcine reproductive and respiratory syndrome virus in the lungs of pigs through adjuvanted nanotechnology-based vaccination. Int. J. Nanomed. 2014;9:1519–1535. doi: 10.2147/IJN.S59924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Wu Y., Lee L.J., Torrelles J.B., Renukaradhya G.J. Adjuvanted poly(lactic-co-glycolic) acid nanoparticle-entrapped inactivated porcine reproductive and respiratory syndrome virus vaccine elicits cross-protective immune response in pigs. Int. J. Nanomed. 2014;9:679–694. doi: 10.2147/IJN.S56127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botner A., Strandbygaard B., Sorensen K.J., Have P., Madsen K.G., Madsen E.S., Alexandersen S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 1997;141:497–499. doi: 10.1136/vr.141.19.497. [DOI] [PubMed] [Google Scholar]
- Chand R.J., Trible B.R., Rowland R.R. Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr. Opin. Virol. 2012;2:256–263. doi: 10.1016/j.coviro.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Charerntantanakul W. Adjuvants for porcine reproductive and respiratory syndrome virus vaccines. Vet. Immunol. Immunopathol. 2009;129:1–13. doi: 10.1016/j.vetimm.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Christopher-Hennings J., Holler L.D., Benfield D.A., Nelson E.A. Detection and duration of porcine reproductive and respiratory syndrome virus in semen, serum, peripheral blood mononuclear cells, and tissues from Yorkshire, Hampshire, and Landrace boars. J. Vet. Diagn. Invest. 2001;13:133–142. doi: 10.1177/104063870101300207. [DOI] [PubMed] [Google Scholar]
- Deville S., Arous J.B., Ionkoff G., Bertrand F., Kukushkin S., Baybikov T., Borisov V., Dupuis L. Load reduction in live PRRS vaccines using oil and polymer adjuvants. Proc. Vaccinol. 2012;6:134–140. doi: 10.1016/j.provac.2012.04.018. (5th Vaccine and ISV Global Annual Congress, www.elsevier.com/locate/procedia) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldhof M.F., Vanhee M., Van Breedam W., Van Doorsselaere J., Karniychuk U.U., Nauwynck H.J. Comparison of the efficacy of autogenous inactivated Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) vaccines with that of commercial vaccines against homologous and heterologous challenges. BMC Vet. Res. 2012;8:182. doi: 10.1186/1746-6148-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbur P.G., Paul P.S., Frey M.L., Landgraf J., Eernisse K., Meng X.J., Lum M.A., Andrews J.J., Rathje J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- Holtkamp D., Kliebenstein J. 2011. PRRS Costs Industry $664 Million Annually Pork Checkoff Study.http://www.pork.org/News/1265/PRRSCostsIndustry664Million.aspx [Google Scholar]
- Kim W.I., Lee D.S., Johnson W., Roof M., Cha S.H., Yoon K.J. Effect of genotypic and biotypic differences among PRRS viruses on the serologic assessment of pigs for virus infection. Vet. Microbiol. 2007;123:1–14. doi: 10.1016/j.vetmic.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kimman T.G., Cornelissen L.A., Moormann R.J., Rebel J.M., Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009;27:3704–3718. doi: 10.1016/j.vaccine.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Kwang J., Zuckermann F., Ross G., Yang S., Osorio F., Liu W., Low S. Antibody and cellular immune responses of swine following immunisation with plasmid DNA encoding the PRRS virus ORF’s 4, 5, 6 and 7. Res. Vet. Sci. 1999;67:199–201. doi: 10.1053/rvsc.1998.0291. [DOI] [PubMed] [Google Scholar]
- Labarque G., Reeth K.V., Nauwynck H., Drexler C., Van Gucht S., Pensaert M. Impact of genetic diversity of European-type porcine reproductive and respiratory syndrome virus strains on vaccine efficacy. Vaccine. 2004;22:4183–4190. doi: 10.1016/j.vaccine.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lager K.M., Mengeling W.L., Brockmeier S.L. Evaluation of protective immunity in gilts inoculated with the NADC-8 isolate of porcine reproductive and respiratory syndrome virus (PRRSV) and challenge-exposed with an antigenically distinct PRRSV isolate. Am. J. Vet. Res. 1999;60:1022–1027. [PubMed] [Google Scholar]
- Lee J.A., Kwon B., Osorio F.A., Pattnaik A.K., Lee N.H., Lee S.W., Park S.Y., Song C.S., Choi I.S., Lee J.B. Protective humoral immune response induced by an inactivated porcine reproductive and respiratory syndrome virus expressing the hypo-glycosylated glycoprotein 5. Vaccine. 2014;32:3617–3622. doi: 10.1016/j.vaccine.2014.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xue C., Wang L., Chen X., Chen F., Cao Y. Genomic analysis of two Chinese strains of porcine reproductive and respiratory syndrome viruses with different virulence. Virus Genes. 2010;40:374–381. doi: 10.1007/s11262-010-0453-z. [DOI] [PubMed] [Google Scholar]
- Li X., Galliher-Beckley A., Nietfeld J.C., Faaberg K.S., Shi J. Montanide TM Gel01 ST adjuvant enhances PRRS modified live vaccine efficacy by regulating porcine humoral and cellular immune responses. World J. Vaccines. 2013;3:1–9. doi: 10.4236/wjv.2013.31001. [DOI] [Google Scholar]
- Lopez O.J., Osorio F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 2004;102:155–163. doi: 10.1016/j.vetimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Martelli P., Gozio S., Ferrari L., Rosina S., De Angelis E., Quintavalla C., Bottarelli E., Borghetti P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: clinical protection and cell-mediated immunity. Vaccine. 2009;27:3788–3799. doi: 10.1016/j.vaccine.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Misinzo G., Delputte P.L., Meerts P., Drexler C., Nauwynck H.J. Efficacy of an inactivated PRRSV vaccine: induction of virus-neutralizing antibodies and partial virological protection upon challenge. Adv. Exp. Med. Biol. 2006;581:449–454. doi: 10.1007/978-0-387-33012-9_81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh M.P., Genzow M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS) Vaccine. 2011;29:8192–8204. doi: 10.1016/j.vaccine.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Nelsen C.J., Murtaugh M.P., Faaberg K.S. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilubol D., Platt K.B., Halbur P.G., Torremorell M., Harris D.L. The effect of a killed porcine reproductive and respiratory syndrome virus (PRRSV) vaccine treatment on virus shedding in previously PRRSV infected pigs. Vet. Microbiol. 2004;102:11–18. doi: 10.1016/j.vetmic.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Orynbayev M., Belloussov V., Mambetaliyev M., Kopochenya A.A., Burashev Ye., Kerimbayev A., Kopeyev S., Mamadaliyev S., 2010. Identification of porcine reproductive and respiratory syndrome virus of North American genotype in Republic of Kazakhstan (in Russian). In Proceedings of VII scientific and practice conference: Molecular diagnostic-2010, Moscow, Russia, 24–26 November 2010 159–162.
- Osorio F.A., Galeota J.A., Nelson E., Brodersen B., Doster A., Wills R., Zuckermann F., Laegreid W.W. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology. 2002;302:9–20. doi: 10.1006/viro.2002.1612. [DOI] [PubMed] [Google Scholar]
- Parker R., Deville S., Dupuis L., Bertrand F., Aucouturier J. Adjuvant formulation for veterinary vaccines: Montanide™ Gel safety profile. Proc. Vaccinol. 2008;1:140–147. (2nd Vaccine Global Congress, Boston, 2008 www.elsevier.com/locate/procedia) [Google Scholar]
- Reed L.J., Muench L. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27(3):493–497. [Google Scholar]
- Renukaradhya G.J., Meng X.J., Calvert J.G., Roof M., Lager K.M. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: current status and future direction. Vaccine. 2015;33:3065–3072. doi: 10.1016/j.vaccine.2015.04.102. [DOI] [PubMed] [Google Scholar]
- Renukaradhya G.J., Meng X.J., Calvert J.G., Roof M., Lager K.M. Live porcine reproductive and respiratory syndrome virus vaccines: current status and future direction. Vaccine. 2015;33:4069–4080. doi: 10.1016/j.vaccine.2015.06.092. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Bautista E.M., Goyal S.M., Molitor T.W., Murtaugh M.P., Morrison R.B., Benfield D.A., Collins J.E. Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and 10-week-old pigs. J. Vet. Diagn. Invest. 1994;6:3–12. doi: 10.1177/104063879400600102. [DOI] [PubMed] [Google Scholar]
- Scortti M., Prieto C., Alvarez E., Simarro I., Castro J.M. Failure of an inactivated vaccine against porcine reproductive and respiratory syndrome to protect gilts against a heterologous challenge with PRRSV. Vet. Rec. 2007;161:809–813. [PubMed] [Google Scholar]
- Spellberg B., Edwards J.E., Jr. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- Tabynov K.K., Mambetaliyev M., Azhibayev A.Z., Orynbayev M.B., Zaitsev V.L., Kopochenya A.A., Azanbekova A.A., Sansyzbay A.R. Isolation of the European genotype porcine reproductive and respiratory syndrome virus (PRRSV) in Kazakhstan. International PRRS Symposium; Beijing, China 20–22 May 2013; 2013. p. 114. [Google Scholar]
- Tabynov K., Sansyzbay A., Sandybayev N., Mambetaliyev M. The pathogenicity of swan derived H5N1 virus in birds and mammals and its gene analysis. Virol. J. 2014;11:207. doi: 10.1186/s12985-014-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabynov K., Ryskeldinova S., Sansyzbay A. An influenza viral vector Brucella abortus vaccine induces good cross-protection against Brucella melitensis infection in pregnant heifers. Vaccine. 2015;33:3619–3623. doi: 10.1016/j.vaccine.2015.06.045. [DOI] [PubMed] [Google Scholar]
- Tabynov K.K. BEI-inactivated PRRS vaccine adjuvanted with MontanideTM Gel 01 ST elicits virus specific cross-protective immune responses in piglets. J. Antivir. Antiretrovir. 2014;6:2. doi: 10.4172/1948-5964.S1022. [DOI] [Google Scholar]
- Thanawongnuwech R., Suradhat S. Taming PRRSV: revisiting the control strategies and vaccine design. Virus Res. 2010;154:133–140. doi: 10.1016/j.virusres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Vanhee M., Delputte P.L., Delrue I., Geldhof M.F., Nauwynck H.J. Development of an experimental inactivated PRRSV vaccine that induces virus-neutralizing antibodies. Vet. Res. 2009;40:63. doi: 10.1051/vetres/2009046. [DOI] [PubMed] [Google Scholar]
- Yang L., Frey M.L., Yoon K.J., Zimmerman J.J., Platt K.B. Categorization of North American porcine reproductive and respiratory syndrome viruses: epitopic profiles of the N, M, GP5 and GP3 proteins and susceptibility to neutralization. Arch. Virol. 2000;145:1599–1619. doi: 10.1007/s007050070079. [DOI] [PubMed] [Google Scholar]
- Zimmerman J.J., Benfield D., Dee S., Murtaugh M., Stadejek T., Stevenson G.W., Torremorell M. tenth edition. John Wiley & Sons, Inc.; 2012. Porcine Reproductive and Respiratory Syndrome Virus (Porcine Arterivirus). Diseases of Swine. [Google Scholar]
- Zuckermann F.A., Garcia E.A., Luque I.D., Christopher-Hennings J., Doster A., Brito M., Osorio F. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet. Microbiol. 2007;123:69–85. doi: 10.1016/j.vetmic.2007.02.009. [DOI] [PubMed] [Google Scholar]