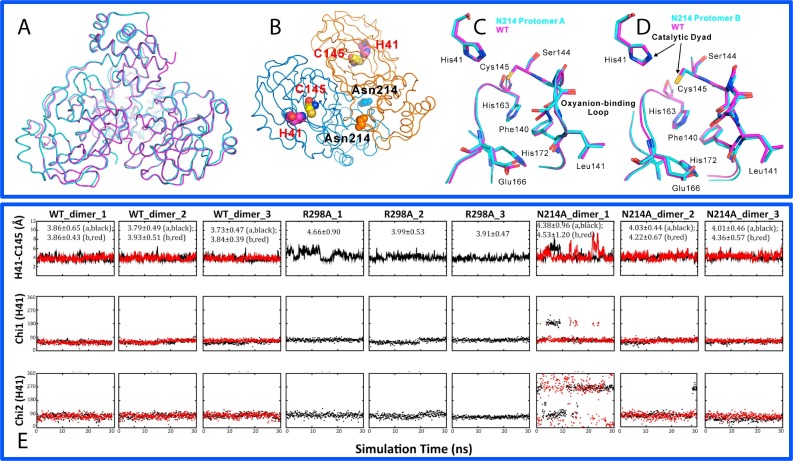
Fig. 4.
Dynamically-driven inactivation of the catalytic machinery by the N214A mutation on the extra domain. (A)Overall superimposition of the dimeric Asn214Ala (violet) and WT (cyan) structures. (B) Dimeric structure of the SARS 3C-like protease showing the catalytic dyad His41-Cys145 located on the cleft of domain I (brown) and domain II (blue) of the chymotrypsin fold, as well as Asn214 on the extra domain. Superimpositions of the catalytically critical residues of WT (violet) and Asn214Ala (cyan) for protomers A (C) and B (D) respectively. (E) Dynamic behavior of the catalytic dyad as reflected by the time-trajectories of the distance between NE2 of His41 and SG of Cys145 atoms, of the Chi1 dihedral angle of His41 and of the Chi2 dihedral angle of His41 of WT; R298A and N214A.