Figure 4.
Interface Residues between NTD and Domain C Are Involved in Cholesterol Transfer from NPC2 to NPC1
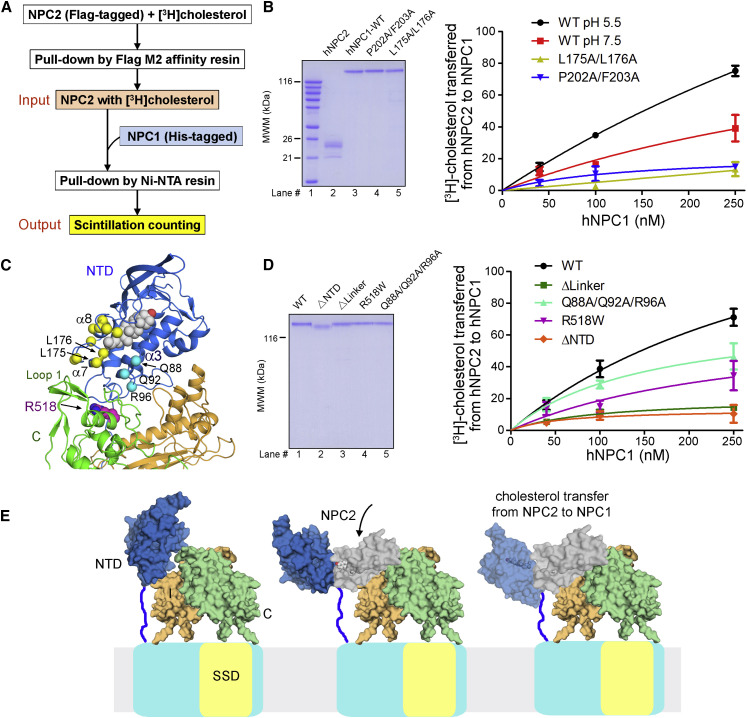
(A) The simplified protocol for an in vitro cholesterol transfer assay using purified full-length (FL) hNPC1 and hNPC2. Please refer to the Supplemental Experimental Procedures for details.
(B) Validation of the assay system with reported conditions and mutations. Left: the purified hNPC2 and hNPC1 variants used in the assay. Right: Ala substitution of the previously identified residues for sterol binding or transfer markedly reduced cholesterol transfer from NPC2 to FL NPC1 in our assay (Kwon et al., 2009). For each data point, the reading of the negative control, whereby NPC1 was omitted in the pull-down experiment, was subtracted from the output, and the resulted reading was normalized against the input and presented in percentage. Each data point is the average of three independent experiments. Error bars represent SD.
(C) Structural mapping of the NTD residues that are important for cholesterol transfer between NPC2 and NPC1(NTD). A cholesterol molecule, shown as silver spheres, was modeled based on the crystal structure of cholesterol-bound NTD (PDB: 3GKI). The Cα atoms of the previously characterized functional residues (Wang et al., 2010) and the ones identified in this study are shown as yellow and cyan spheres, respectively. Note that these residues are in the vicinity of the domain C residue Arg518, which is disease related and important for NPC2 recruitment (Deffieu and Pfeffer, 2011).
(D) The cholesterol transfer activities of NPC1 variants designed on the basis of the EM structure. Gln88/Gln92/Arg96 and Arg518 are on the interface between NTD and domain C (please refer to the inset of Figure 3D).
(E) The structure and biochemical characterizations suggest that sterol transfer between NPC2 and NPC1 necessitates proper accommodation of NPC2 by NPC1(NTD) and NPC1-C. Shown here are domain models, whose relative orientations and scales may be different from the real structure. Arrival of cholesterol-bound NPC2 may dislodge NTD from its interactions with domains C and I. NPC2 may be oriented by both the NTD and NPC1-C for the hydrophobic handoff of cholesterol to the NTD.