Figure 5.
Examination of the Interaction between Cleaved Glycoprotein of Ebola Virus (EBOV-GPcl) and FL NPC1
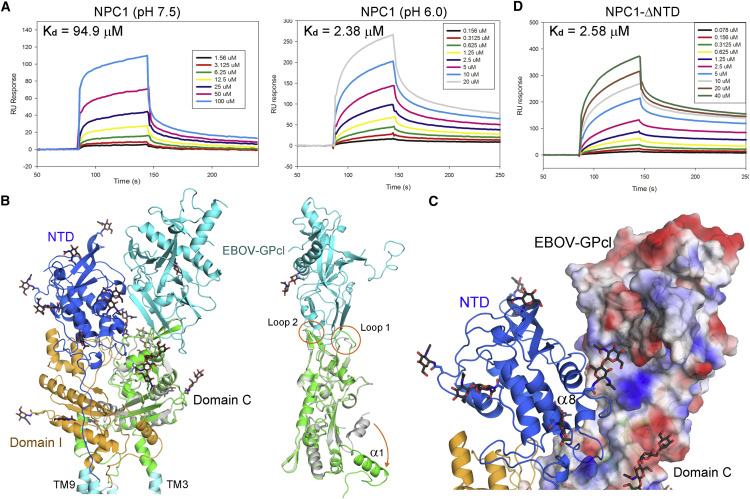
(A) The interaction between EBOV-GPcl and NPC1 is pH dependent. The binding affinity is measured using SPR.
(B) Docking of the domain C-GPcl complex (PDB: 5F1B) to the EM structure of hNPC1. Right: conformational changes of domain C. Note the pronounced rearrangement of helix α1 of domain C between the crystal structure of isolated domain (silver) and the EM structure of FL protein (green).
(C) The docking model suggested a potential interface between NPC1(NTD) and EBOV-GPcl. The domain C-GPcl1 complex is shown as semi-transparent surface electrostatic potential calculated in PyMol.
(D) The NTD-deleted hNPC1 binds to EBOV-GPcl with similar affinity as FL protein at pH 6.0.