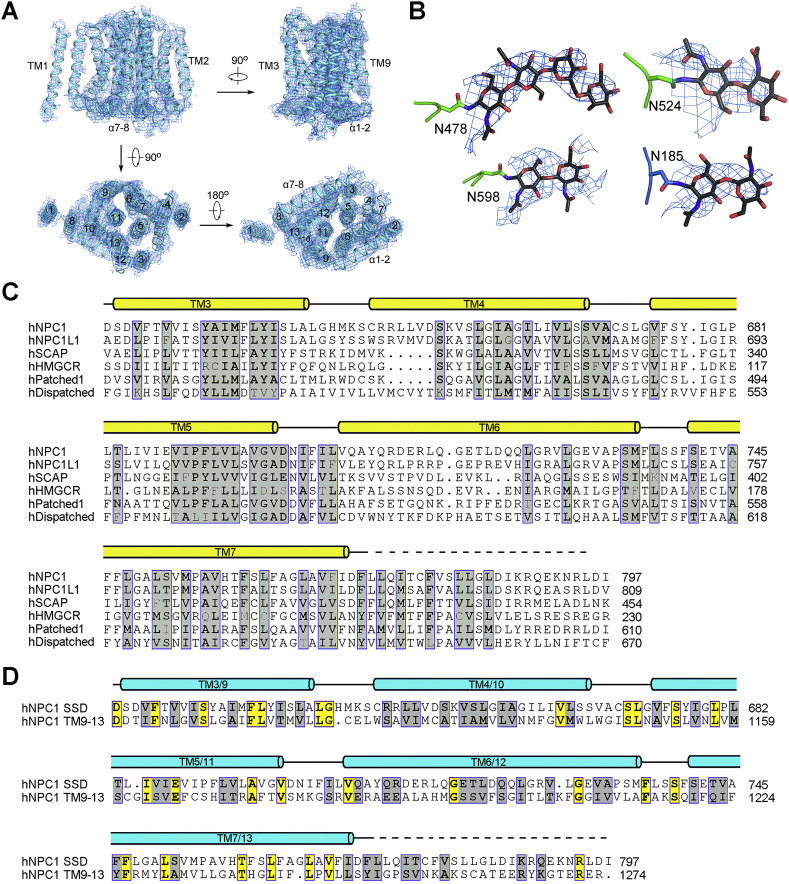
Figure S3.
TM3–7 Constitute the Sterol-Sending Domain, Related to Figure 2
(A) The EM map for the transmembrane domain of hNPC1.
(B) Representative densities of glycosylation sites in NTD and domain C. The maps were generated in PyMol and contoured at 5σ.
(C) Sequence comparison of the SSDs from human proteins involved in cholesterol metabolism or signaling. The UniProt ID numbers for the aligned human sequences are: hNPC1: “UniProt: O15118”; hNPC1L1: “UniProt: Q9UHC9”; hSCAP (SREBP cleavage activating protein): “UniProt: Q12770”; hHMGCR (3-hydroxy-3-methylglutaryl-CoA reductase): “UniProt: P04035”; hPatched1: “UniProt: Q13635”; hDispatched1: “UniProt: Q96F81”.
(D) TMs 9-13 of hNPC1 share sequence similarity with SSD. Shown here is the sequence comparison of SSD to the corresponding segments (TMs 9-13) in the second repeat of hNPC1. Invariant and conserved residues are shaded yellow and gray, respectively. Due to moderate resolution of the TMD, the boundaries of TM segments shown above the sequences may not be accurate.