Figure S5.
Structure-Invoked Questions on the Sterol Transfer from NPC1(NTD) to Membrane, Related to Figure 4
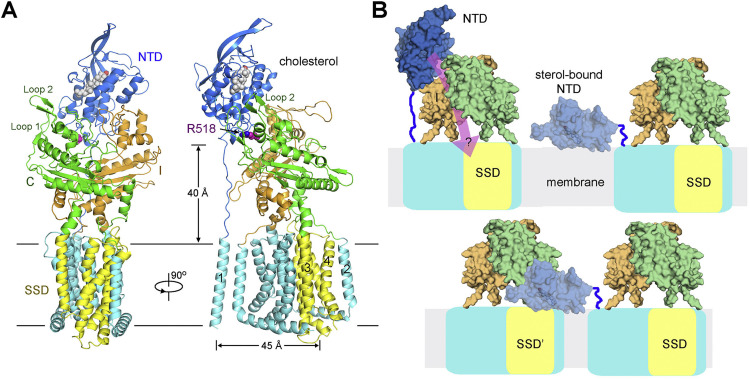
(A) The NTD may not be able to reach SSD in the same molecule when it bends toward the membrane. A cholesterol molecule is modeled into the pocket of NTD based on the crystal structure of cholesterol-bound hNPC1(NTD) (PDB: 3GKI). Arg518 is shown as magenta spheres. The SSD is colored yellow. It is noteworthy that the NTD is on the opposite side of SSD in the structure. The distance between TM1 and the closest TM in SSD is > 45 Å while the linker between NTD and TM1 is about 40 Å. Furthermore, the relative stability of domains C and I with their respective anchoring TMD repeats may represent a spatial block to impede the placement of NTD to the proximity of SSD in the same protein molecule for direct delivery of the NTD-bound cholesterol to SSD.
(B) The molecular mechanism of cholesterol transfer from NPC1(NTD) to the membrane or SSD remains enigmatic. Shown here are speculative models. The pink arrow in the left panel of the upper row indicates the question of whether cholesterol can go from NTD through domains C and I to SSD if NPC1 functions like a RND transporter. Alternatively, NTD may directly deliver the bound cholesterol to membrane (upper right) or to the SSD of an adjacent NPC1 protein (lower row). However, the upper model cannot explain the loss of binding to cholesterol analog due to mutations in SSD unless SSD functions in later steps in cholesterol egress. The “transfer in trans” model in the bottom row is completely hypothetical especially when there is no evidence to support oligomerization of NPC1. The mechanism of cholesterol egress awaits further investigations.